Question: 2. A silver wire dipped in 0.1 M HCl solution saturated with AgCl develops oxidation potential of -0.25V. EAg/Ag- = -0.799V, the Ksp of
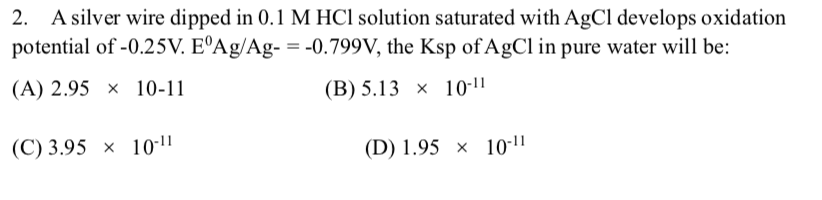
2. A silver wire dipped in 0.1 M HCl solution saturated with AgCl develops oxidation potential of -0.25V. EAg/Ag- = -0.799V, the Ksp of AgCl in pure water will be: (A) 2.95 x 10-11 (B) 5.13 x 10-11 (C) 3.95 x 10-11 (D) 1.95 10-11
Step by Step Solution
3.38 Rating (154 Votes )
There are 3 Steps involved in it
Ecell Ecell 00592 log Ag TA Tq m 25V 0799V ... View full answer

Get step-by-step solutions from verified subject matter experts


