Question: 2. A simple non premixed stoichiometric Propane (CH) and air flame is assumed to have an infinitely fast reaction to CO and H0 (assume
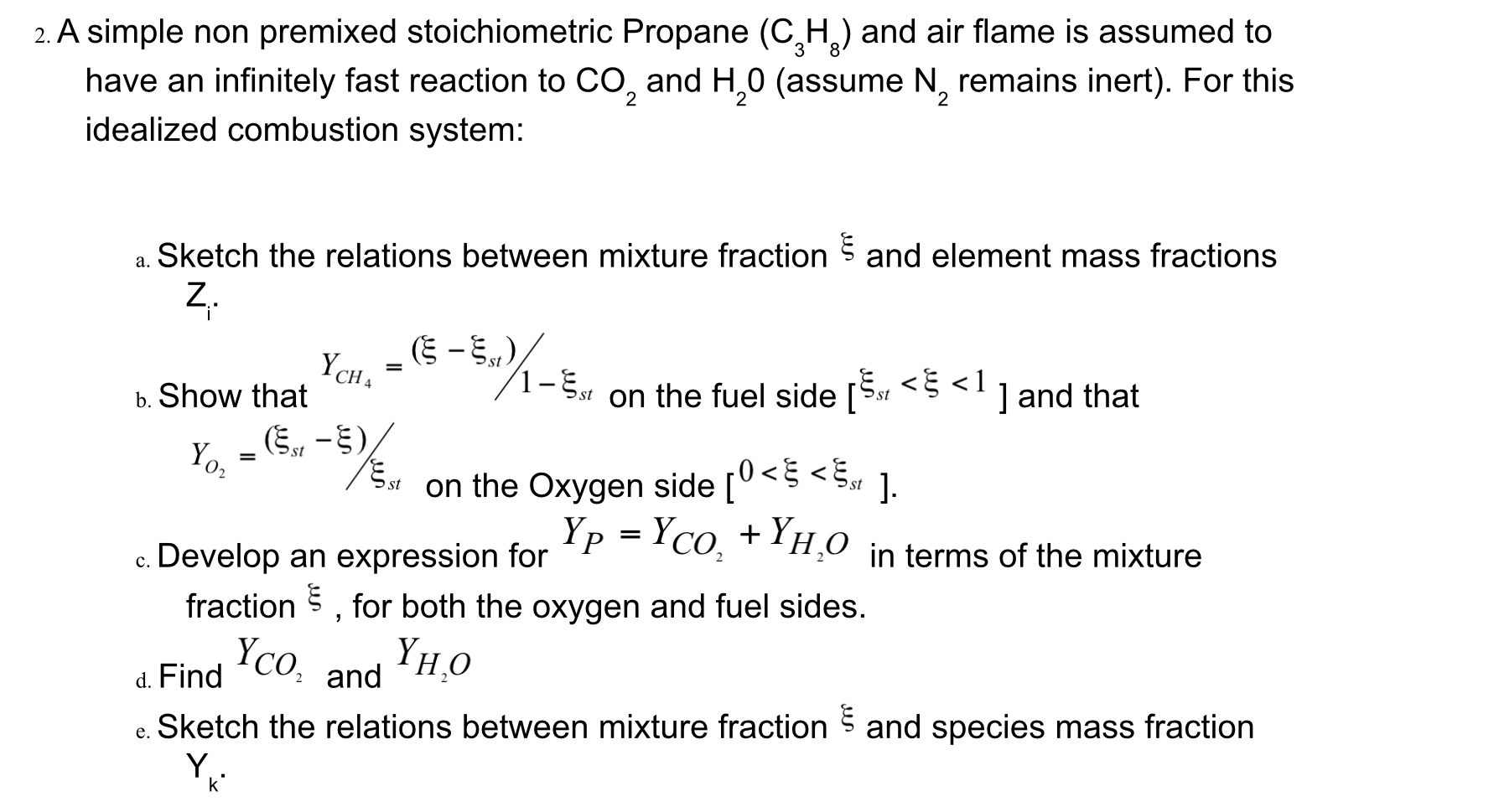
2. A simple non premixed stoichiometric Propane (CH) and air flame is assumed to have an infinitely fast reaction to CO and H0 (assume N, remains inert). For this idealized combustion system: 2 2 2 a. Sketch the relations between mixture fraction and element mass fractions Z. b. Show that Est YCH e. _(-5,)/ = " -Est [st /1-st on the fuel side [ST <
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


