Question: 2. A student has two compounds in two separate bottles but with no labels on either one. One is an alkane, octane (C8H18); the other

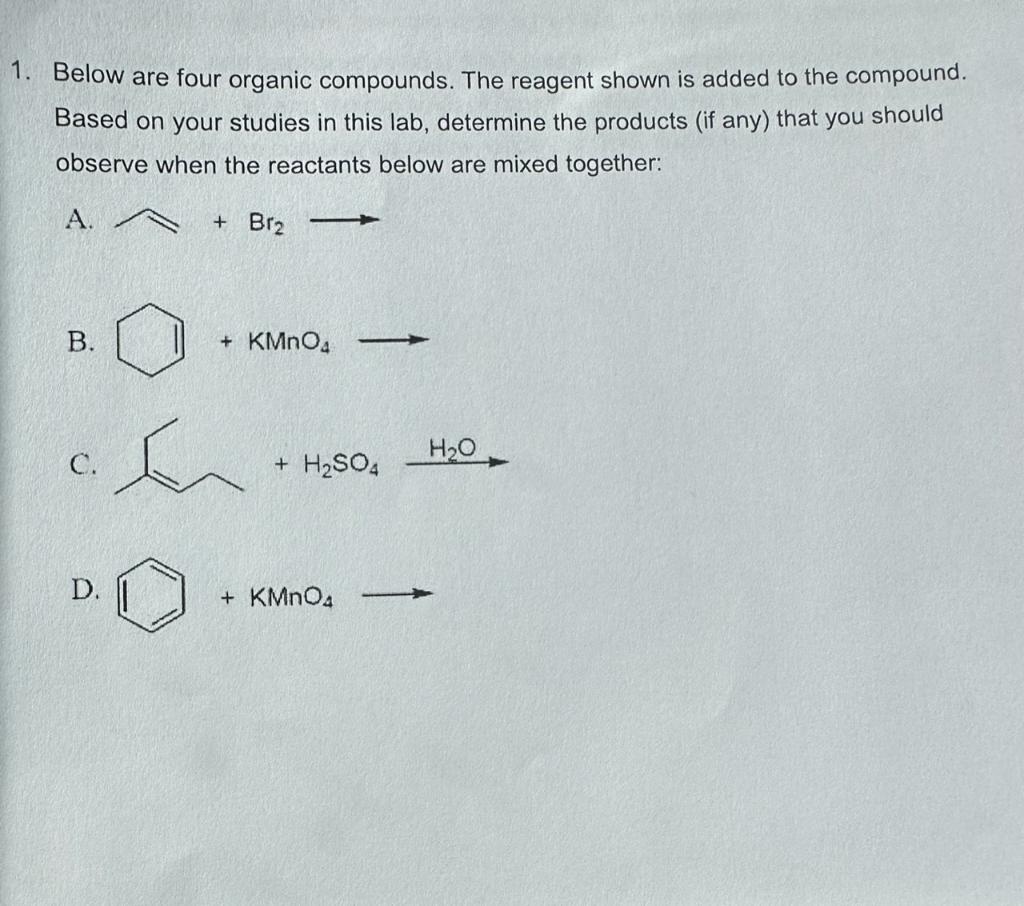
2. A student has two compounds in two separate bottles but with no labels on either one. One is an alkane, octane (C8H18); the other is 1-hexene (C6H12), an alkene. Based on your observations in this experiment, what should you see in the following tests? Octane 1-Hexene A. Water solubility B. Ligroin solubility C. Density versus water D. Bromine test E. Permanganate test 3. An unknown compound, believed to be a hydrocarbon, showed the following behavior: no heat or color appeared when sulfuric acid was added; permanganate solution remained purple; and the red color of bromine solution was lost only after a catalyst was added. From the compounds below, circle the ONE that fits the observations. Below are four organic compounds. The reagent shown is added to the compound. Based on your studies in this lab, determine the products (if any) that you should observe when the reactants below are mixed together: A. +Br2 B. C. D
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


