Question: 2. An inventor proposes using a SHE (standard hydrogen electrode) in a new battery for smartphones that also removes toxic carbon monoxide from the air:
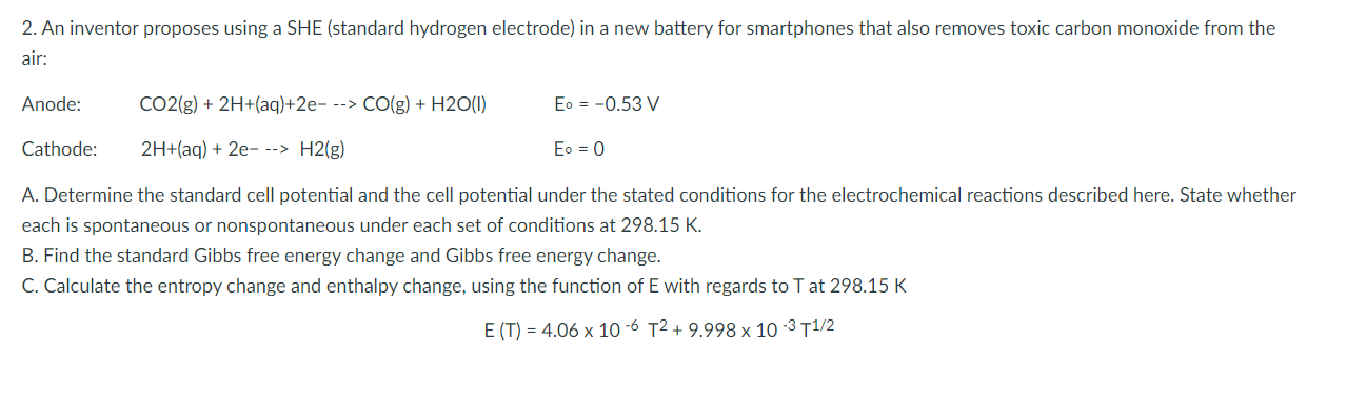
2. An inventor proposes using a SHE (standard hydrogen electrode) in a new battery for smartphones that also removes toxic carbon monoxide from the air: Anode: CO2(g) + 2H+(aq)+2e- --> CO(g) + H20(1) Eo = -0.53 V Cathode: 2H+(aq) + 2e---> H2(g) Eo = 0 A. Determine the standard cell potential and the cell potential under the stated conditions for the electrochemical reactions described here. State whether each is spontaneous or nonspontaneous under each set of conditions at 298.15 K. B. Find the standard Gibbs free energy change and Gibbs free energy change. C. Calculate the entropy change and enthalpy change, using the function of E with regards to T at 298.15 K E (T) = 4.06 x 10-6 T2 + 9.998 x 10 -3 11/2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


