Question: 2. Answer ALL parts (a) (c). (a) Give definitions, with examples where appropriate, of BOTH of the following terms: (i) Nitrene (ii) Radical Initiator (6
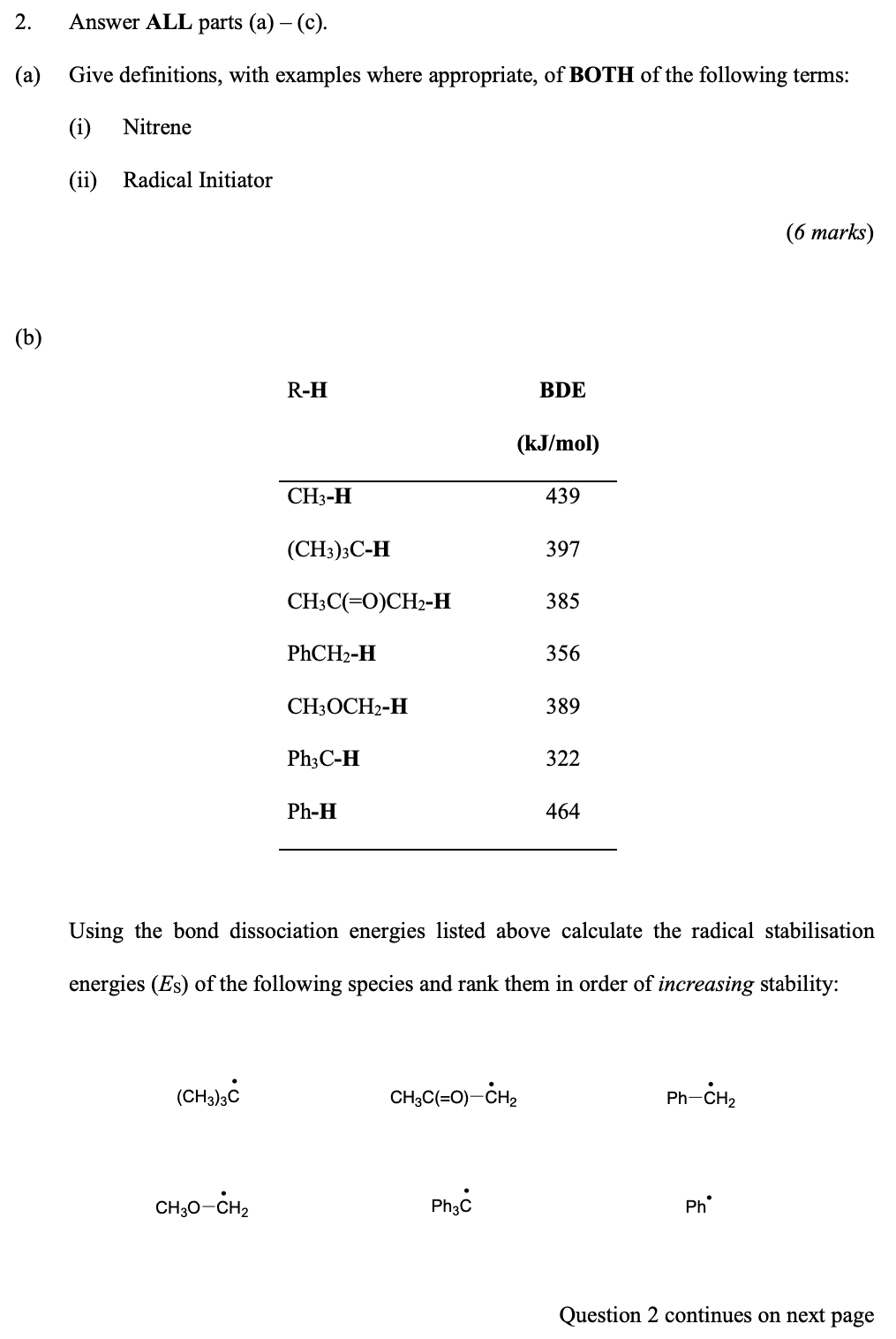
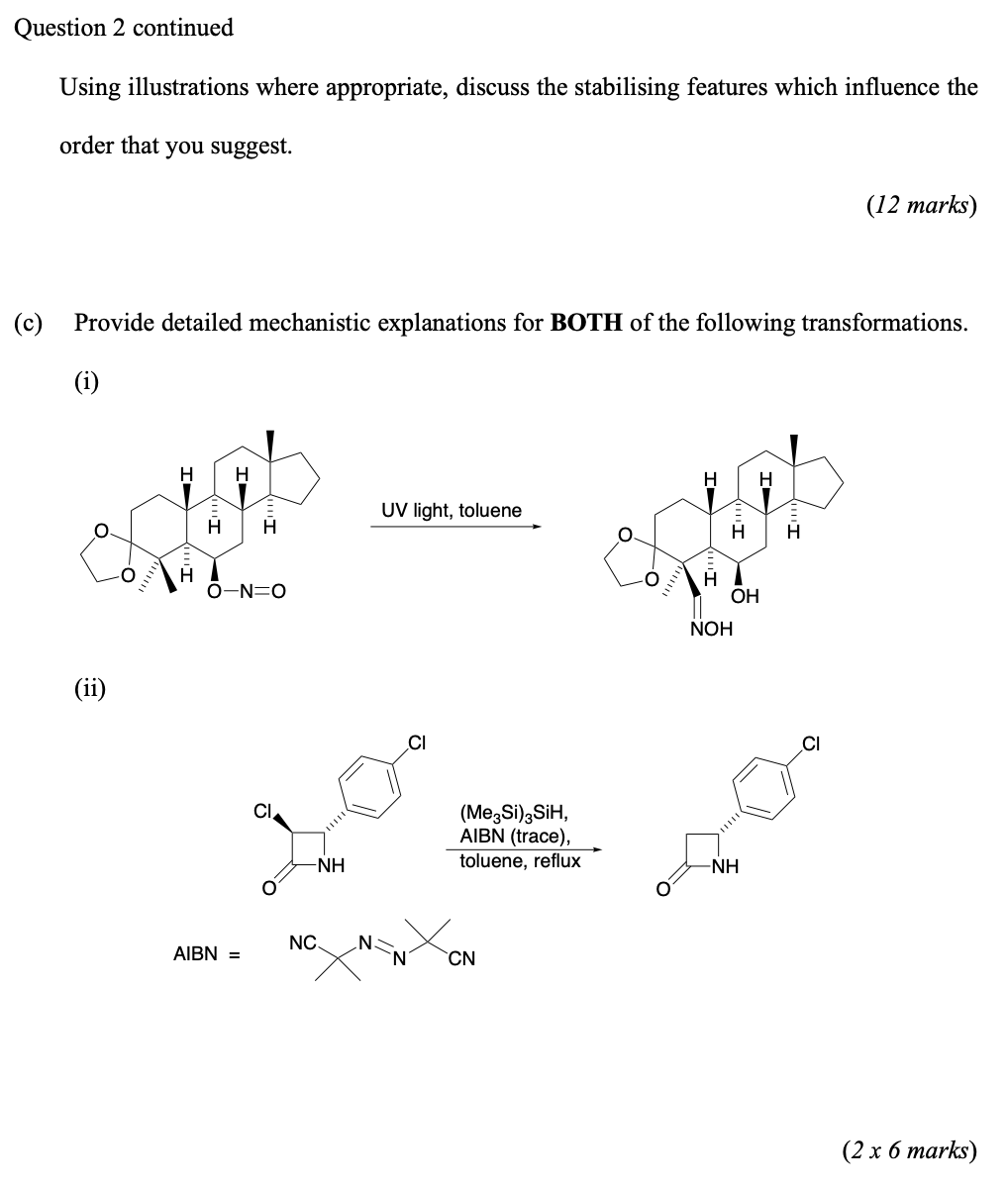
2. Answer ALL parts (a) (c). (a) Give definitions, with examples where appropriate, of BOTH of the following terms: (i) Nitrene (ii) Radical Initiator (6 marks) (b) R-H BDE (kJ/mol) CH3-H 439 (CH3)3C-H 397 CH3C(=O)CH2-H 385 PhCH2-H 356 CH3OCH2-H 389 Ph3C-H 322 Ph-H 464 Using the bond dissociation energies listed above calculate the radical stabilisation energies (Es) of the following species and rank them in order of increasing stability: (CH3)3c CH3C(=O)-CH2 PhCH, CH30-CH2 Phi Ph Question 2 continues on next page Question 2 continued Using illustrations where appropriate, discuss the stabilising features which influence the order that you suggest. (12 marks) (c) Provide detailed mechanistic explanations for BOTH of the following transformations. (i) H H UV light, toluene . H . H 0-N=0 O H OH NOH (ii) CI CI (MezSi) SiH, AIBN (trace), toluene, reflux -NH - NC N. AIBN = CN (2 x 6 marks)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


