Question: 2. By using the Transition State Theory, calculate the pre-exponential factor, A, at 298 K for the following reaction: K K # k1 Br(g) +
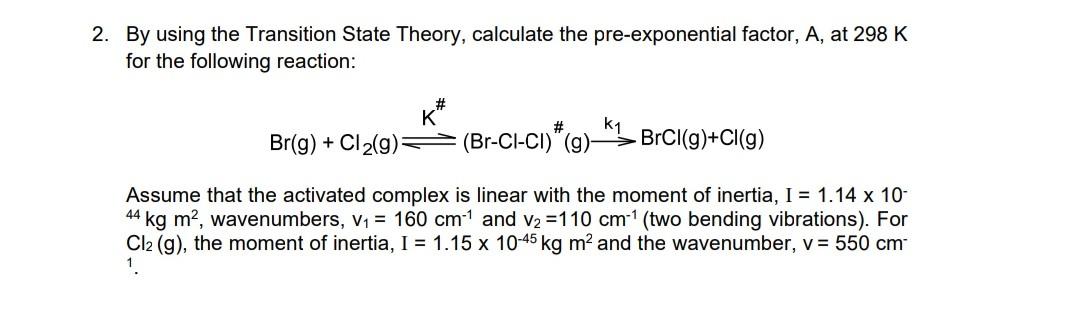
2. By using the Transition State Theory, calculate the pre-exponential factor, A, at 298 K for the following reaction: K K # k1 Br(g) + Cl2(9) (Br-CI-CI)" (g) BrCl(9)+Cl(9) Assume that the activated complex linear with the moment of inertia, I = 1.14 x 10- 44 kg m2, wavenumbers, V1 = 160 cm1 and v2 =110 cm-(two bending vibrations). For Cl2 (g), the moment of inertia, I = 1.15 x 10-45 kg m and the wavenumber, v = 550 cm 1. 2. By using the Transition State Theory, calculate the pre-exponential factor, A, at 298 K for the following reaction: K K # k1 Br(g) + Cl2(9) (Br-CI-CI)" (g) BrCl(9)+Cl(9) Assume that the activated complex linear with the moment of inertia, I = 1.14 x 10- 44 kg m2, wavenumbers, V1 = 160 cm1 and v2 =110 cm-(two bending vibrations). For Cl2 (g), the moment of inertia, I = 1.15 x 10-45 kg m and the wavenumber, v = 550 cm 1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


