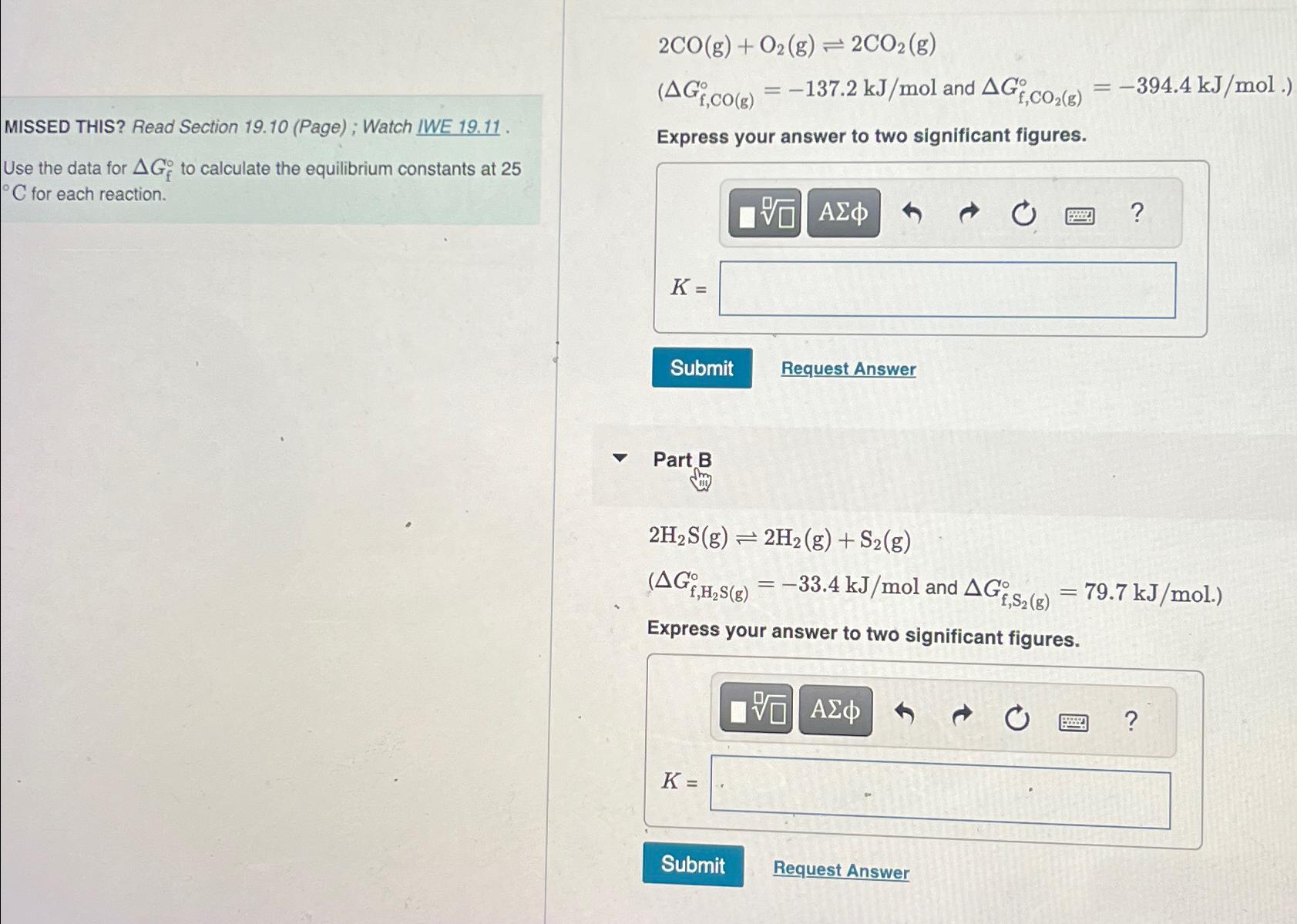
Question: 2 C O ( g ) + O 2 ( g ) 2 C O 2 ( g ) and ( : G f ,
and :
MISSED THIS? Read Section Page; Watch IWE
Use the data for to calculate the equilibrium constants at for each reaction.
Express your answer to two significant figures.
Request Answer
Part B
Express your answer to two significant figures.
Request Answer
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


