Question: 2.) Calculate Vw for a 0.2 Molar NaCl solution (not a cell) at 20C. Note that you are working with a SALT solution that ionizes
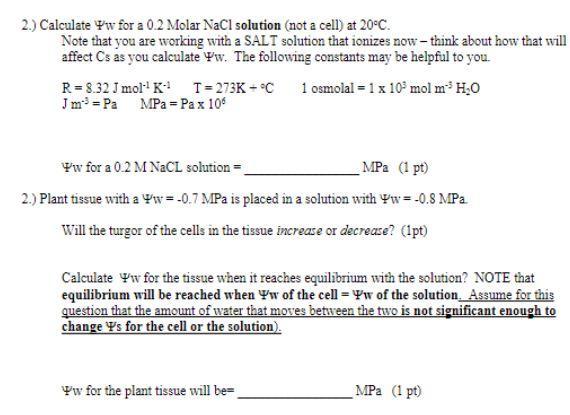
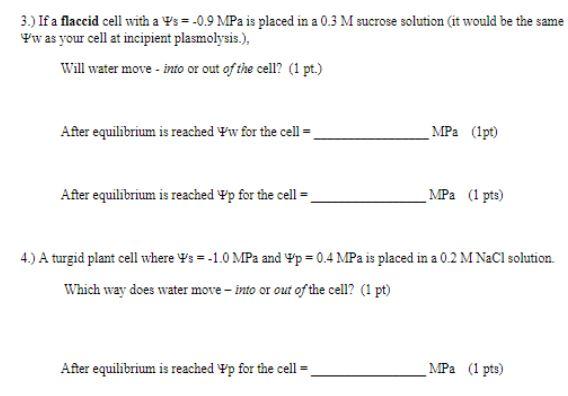
2.) Calculate Vw for a 0.2 Molar NaCl solution (not a cell) at 20C. Note that you are working with a SALT solution that ionizes now - think about how that will affect Cs as you calculate ww. The following constants may be helpful to you. R = 8.32 1 mol K T = 273K - C 1 osmolal = 1 x 102 mol m H.O J m = Pa MPa = Pax 106 w for a 0.2 M NaCl solution = _MPa (1 pt) 2.) Plant tissue with a Ww=-0.7 MPa is placed in a solution with Yw=-0.8 MPa Will the turgor of the cells in the tissue increase or decrease? (Ipt) Calculate w for the tissue when it reaches equilibrium with the solution? NOTE that equilibrium will be reached when yw of the cell = w of the solution. Assume for this question that the amount of water that moves between the two is not significant enough to change Ys for the cell or the solution). w for the plant tissue will be MPa (1 pt) 3.) If a flaccid cell with a Ps= -0.9 MPa is placed in a 0.3 M sucrose solution (it would be the same w as your cell at incipient plasmolysis.), Will water move - into or out of the cell? (1 pt.) After equilibrium is reached w for the cell = MPa (1pt) After equilibrium is reached p for the cell = MPa (1 pts) 4.) A turgid plant cell where Ws=-1.0 MPa and p = 0.4 MPa is placed in a 0.2 M NaCl solution Which way does water move into or out of the cell? (1 pt) After equilibrium is reached Yp for the cell MPa (1 pts)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


