Question: 2. Coffee is commonly decaffeinated using a liquid extraction process in which the coffee beans are soaked in an organic solvent like methylene chloride. Caffeine,
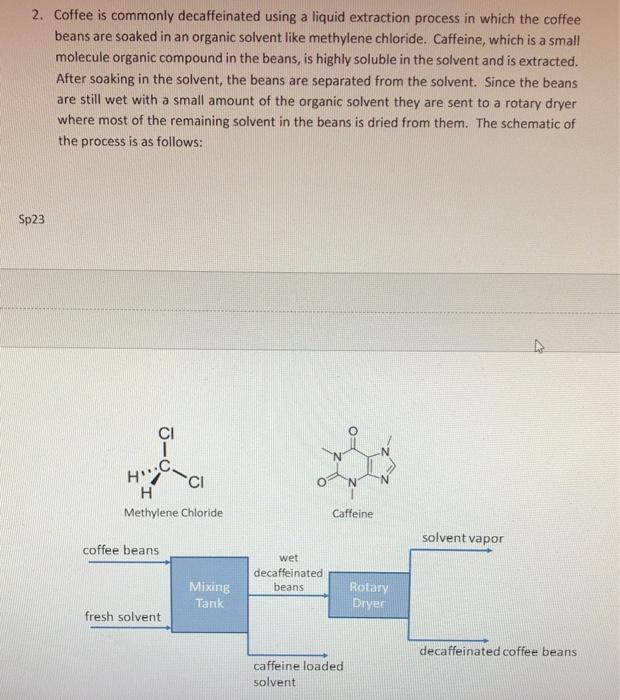

2. Coffee is commonly decaffeinated using a liquid extraction process in which the coffee beans are soaked in an organic solvent like methylene chloride. Caffeine, which is a small molecule organic compound in the beans, is highly soluble in the solvent and is extracted. After soaking in the solvent, the beans are separated from the solvent. Since the beans are still wet with a small amount of the organic solvent they are sent to a rotary dryer where most of the remaining solvent in the beans is dried from them. The schematic of the process is as follows: You job is to analyze this process. You are told the following information: a. 1000kg of coffee beans are loaded into the mixing tank at a time and the beans are 98.5wt% coffee solids and 1.5wt% caffeine. b. 90% of the caffeine in the raw beans is removed with the caffeine loaded solvent stream from the mixing tank. c. The rotary dryer removes 95 wt\% of the remaining solvent in the decaffeinated coffee beans but does not remove any additional caffeine. d. 80% of the solvent fed to the mixing tank leaves in the caffeine loaded solvent stream. e. The solvent evaporated from the beans and leaving the dryer is condensed and collected and is found to 46.80kg of solvent per initial 1000kg bean batch. Answer the following questions: (a) Draw and label a diagram for this system. (b) Perform a degrees of freedom analysis around each possible system boundary you can draw for this system. (c) What is the amount of solvent that must be added to the mixing tank for each 1000 kg batch of coffee beans? (d) What is the solvent content in the final dried decaffeinated beans in units of wt\%
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


