Question: 2) Consider the elementary gas phase reaction 4A+3B3C which is to take place in a flow reactor. Species A and B enter the reactor at

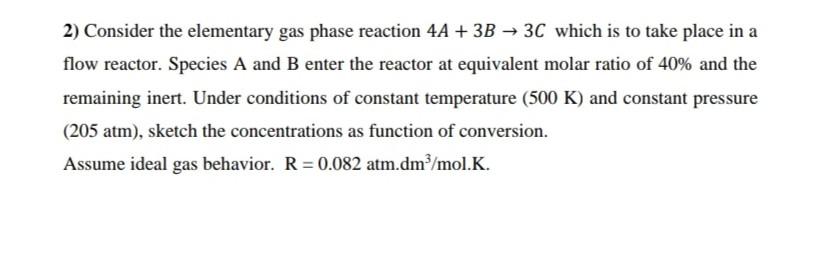
2) Consider the elementary gas phase reaction 4A+3B3C which is to take place in a flow reactor. Species A and B enter the reactor at equivalent molar ratio of 40% and the remaining inert. Under conditions of constant temperature (500K) and constant pressure (205 atm ), sketch the concentrations as function of conversion. Assume ideal gas behavior. R=0.082atm.dm3/mol.K. 2) Consider the elementary gas phase reaction 4A+3B3C which is to take place in a flow reactor. Species A and B enter the reactor at equivalent molar ratio of 40% and the remaining inert. Under conditions of constant temperature (500K) and constant pressure (205 atm), sketch the concentrations as function of conversion. Assume ideal gas behavior. R=0.082atm.dm3/mol.K
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


