Question: 2. Consider the following two molecules with approximately the same size: . H I-0 H - H H H 0.1110 CH3 (a) Why are these
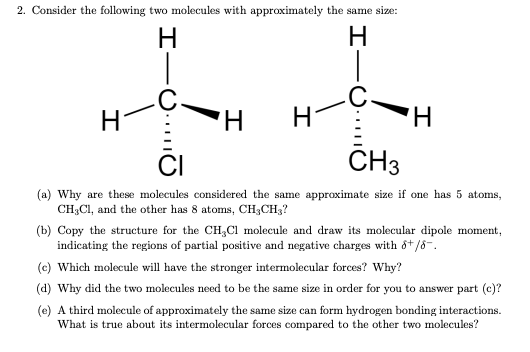
2. Consider the following two molecules with approximately the same size: . H I-0 H - H H H 0.1110 CH3 (a) Why are these molecules considered the same approximate size if one has 5 atoms, CHCI, and the other has 8 atoms, CH2CH3? (b) Copy the structure for the CH CI molecule and draw its molecular dipole moment, indicating the regions of partial positive and negative charges with 8+/8. (c) Which molecule will have the stronger intermolecular forces? Why? (d) Why did the two molecules need to be the same size in order for you to answer part (c)? (e) A third molecule of approximately the same size can form hydrogen bonding interactions. What is true about its intermolecular forces compared to the other two molecules
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


