Question: 2. Does entropy increase or decrease in the following processes? (a) N2+3H22NH3 (b) Identify the circled functional groups and linkages in the compound below. Which
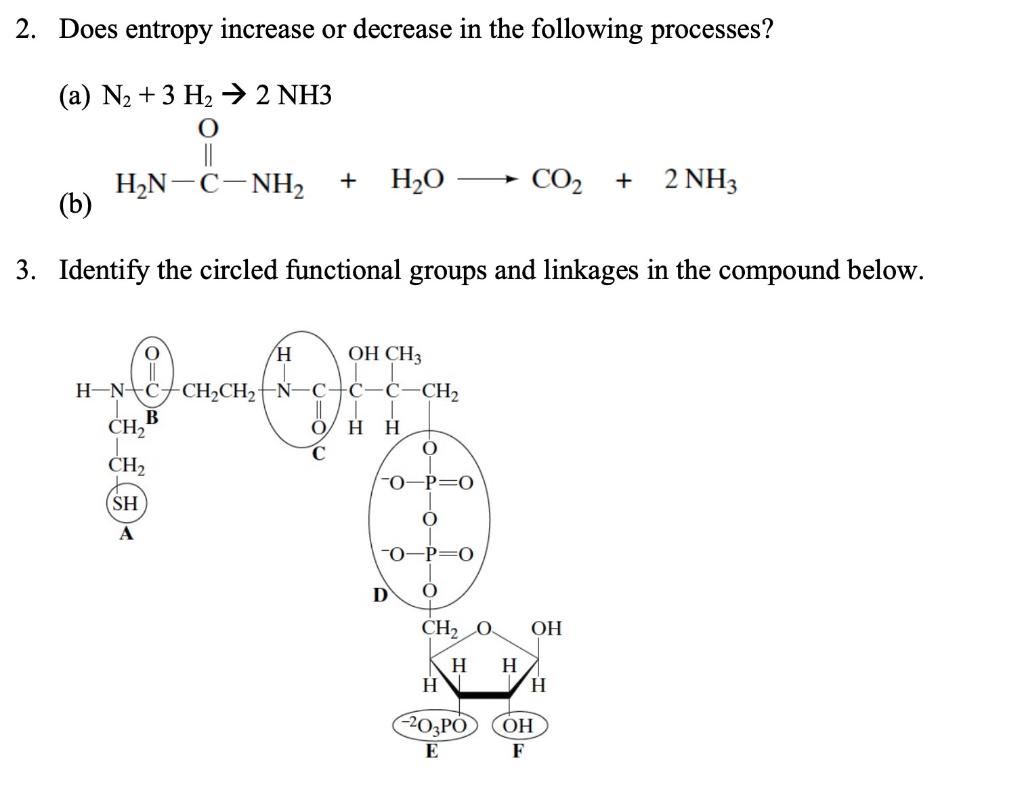
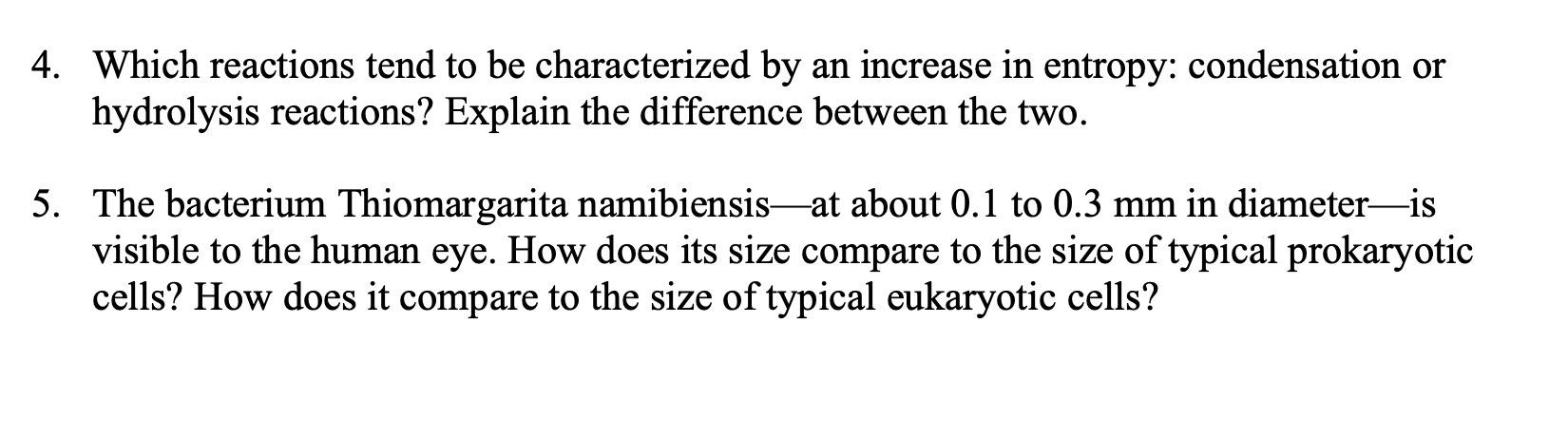
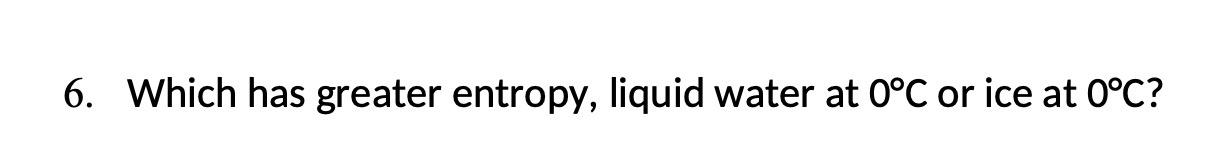
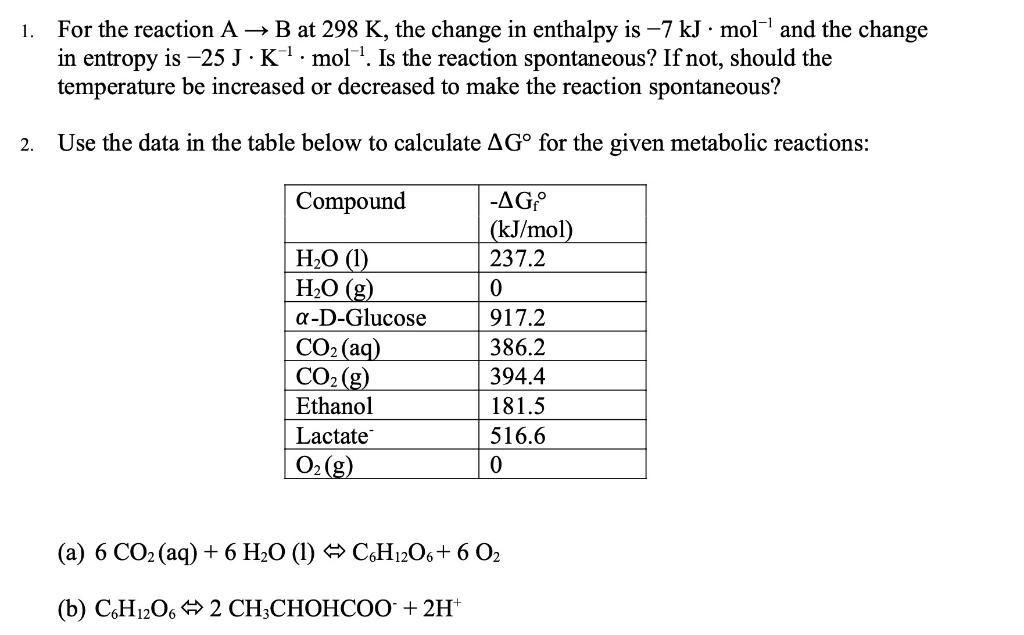
2. Does entropy increase or decrease in the following processes? (a) N2+3H22NH3 (b) Identify the circled functional groups and linkages in the compound below. Which reactions tend to be characterized by an increase in entropy: condensation or hydrolysis reactions? Explain the difference between the two. The bacterium Thiomargarita namibiensis-at about 0.1 to 0.3mm in diameter-is visible to the human eye. How does its size compare to the size of typical prokaryotic cells? How does it compare to the size of typical eukaryotic cells? 6. Which has greater entropy, liquid water at 0C or ice at 0C ? 1. For the reaction AB at 298K, the change in enthalpy is 7kJmol1 and the change in entropy is 25JK1mol1. Is the reaction spontaneous? If not, should the temperature be increased or decreased to make the reaction spontaneous? 2. Use the data in the table below to calculate G for the given metabolic reactions: (a) 6CO2(aq)+6H2O(l)C6H12O6+6O2 (b) C6H12O62CH3CHOHCOO+2H+ 3. Label the following statements true or false: (a) A reaction is said to be spontaneous when it can proceed in either the forward or reverse direction. (b) A spontaneous process always happens very quickly. (c) A nonspontaneous reaction will proceed spontaneously in the reverse direction. 4. Calculate the equilibrium constant for the reaction at pH7.0 and 25C(G=20.9 kJJmol1 ) glucose-1-phosphate +H2O glucose +H2PO4 5. The standard free energy change for the reaction AB is 17.0kJ.mol1. What is the equilibrium constant for the reaction at 25C ? 6. Identify the potential hydrogen bond donors and acceptors in the following molecule
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


