Question: 2. For a first-order elementary reversible reaction (A>B), k is the forward rate constant and k2 is the reverse rate constant. K is the equilibrium
2. For a first-order elementary reversible reaction (A>B), k is the forward rate constant and k2 is the reverse rate constant. K is the equilibrium constant, which is equivalent to (CB)eq/(CA)eq, wherein (C)eq and (CA)eq represent concentrations of B and A at equilibrium state.
- Please derive equation [-d Ca/dt] in terms of CA, ki, k2, Cao and Co. CAo and Cho are initial concentrations of A and B respectively. (10 )
- Please derive K in terms of Co, Ceo and (CA)eg. (5 s)
- Please solve Cs in terms of Cro, (CA)eq, k2, K and 1. (10 1))
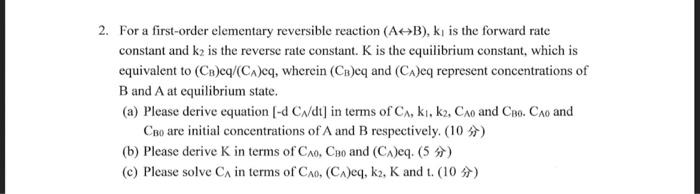
For a first-order elementary reversible reaction (AB),k1 is the forward rate constant and k2 is the reverse rate constant. K is the equilibrium constant, which is equivalent to (CB)eq/(CA)eq, wherein (CB) eq and (CA) eq represent concentrations of B and A at equilibrium state. (a) Please derive equation [dCN/dt] in terms of CA,k1,k2,CA0 and CB0.CA0 and CB0 are initial concentrations of A and B respectively. (10 N) (b) Please derive K in terms of CA0,CB0 and (CA) eq. (5 ) (c) Please solve C in terms of CA,(C) eq, k2,K and t. (10 )
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


