Question: . 2. For the compound below determine: a. How many sp3,sp2 and sp hybridized atoms are present b. The hybridization, at each numbered atom. d.
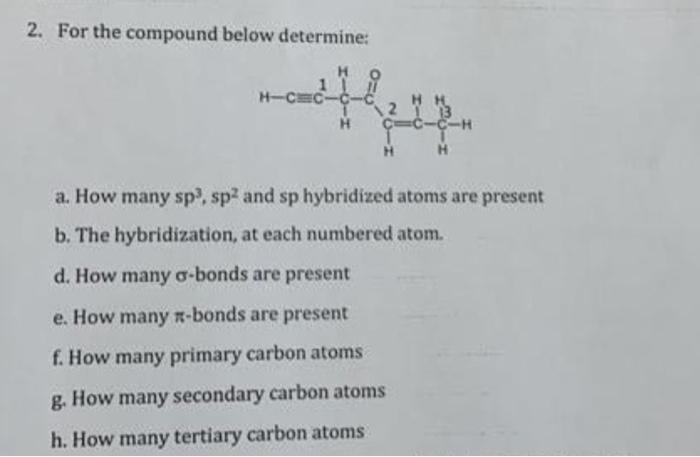
2. For the compound below determine: a. How many sp3,sp2 and sp hybridized atoms are present b. The hybridization, at each numbered atom. d. How many -bonds are present e. How many -bonds are present f. How many primary carbon atoms g. How many secondary carbon atoms h. How many tertiary carbon atoms
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


