Question: 2. Given: the radial wave function of 3p atomic orbital is 4 Z Zr R3p = siva Cal? (60-0?)e0/3,6 = (2)2 ao ao For a
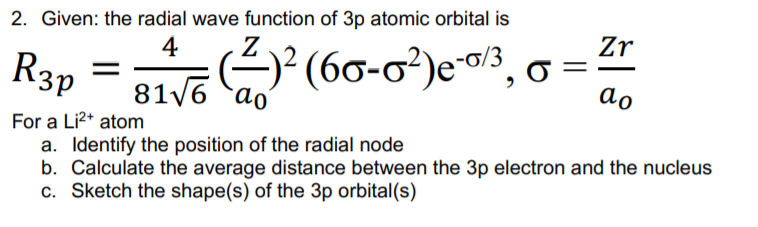
2. Given: the radial wave function of 3p atomic orbital is 4 Z Zr R3p = siva Cal? (60-0?)e0/3,6 = (2)2 ao ao For a Li2+ atom a. Identify the position of the radial node b. Calculate the average distance between the 3p electron and the nucleus C. Sketch the shape(s) of the 3p orbital(s)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


