Question: 2. Identify if these are redox reactions ( 6 marks) a) CuO+COCu+CO2 b) AgCN+CN[Ag(CN)2] c) 2KI+Pb(NO3)22KNO3+PbI2 d) Fe(s)+CuSO4(aa)FeSO4(aa)+Cu(s) e) P4+3NaOH+3H2O3NaH2PO2+PH3 f) NH4CHCl+NH3
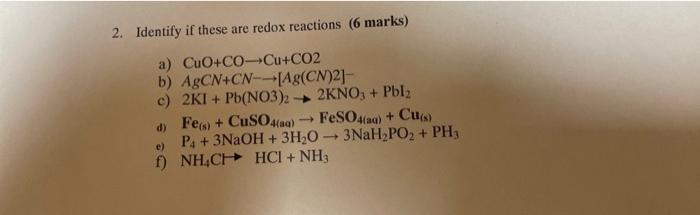
2. Identify if these are redox reactions ( 6 marks) a) CuO+COCu+CO2 b) AgCN+CN[Ag(CN)2] c) 2KI+Pb(NO3)22KNO3+PbI2 d) Fe(s)+CuSO4(aa)FeSO4(aa)+Cu(s) e) P4+3NaOH+3H2O3NaH2PO2+PH3 f) NH4CHCl+NH3
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


