Question: 2. Identify the strongest intermolecular force present in pure samples of the following substances when they exist in the liquid/solid phase: CH3CN SO3 CCI4
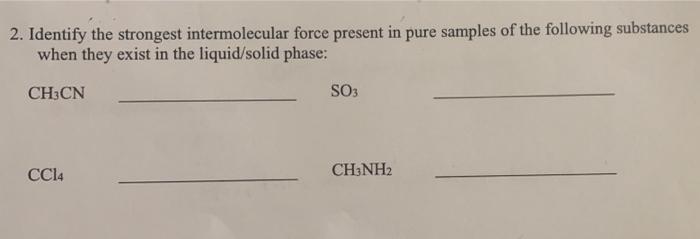
2. Identify the strongest intermolecular force present in pure samples of the following substances when they exist in the liquid/solid phase: CH3CN SO3 CCI4 CH:NH2
Step by Step Solution
There are 3 Steps involved in it
1CH3CN Dipole dipole forces Since CH3CN is much polar its dipole dipole forces are much stronger an... View full answer

Get step-by-step solutions from verified subject matter experts


