Question: 2. If proline is connected directly to a solid phase by an ester bond, the peptide synthesis fails once the second coupled amino acid is
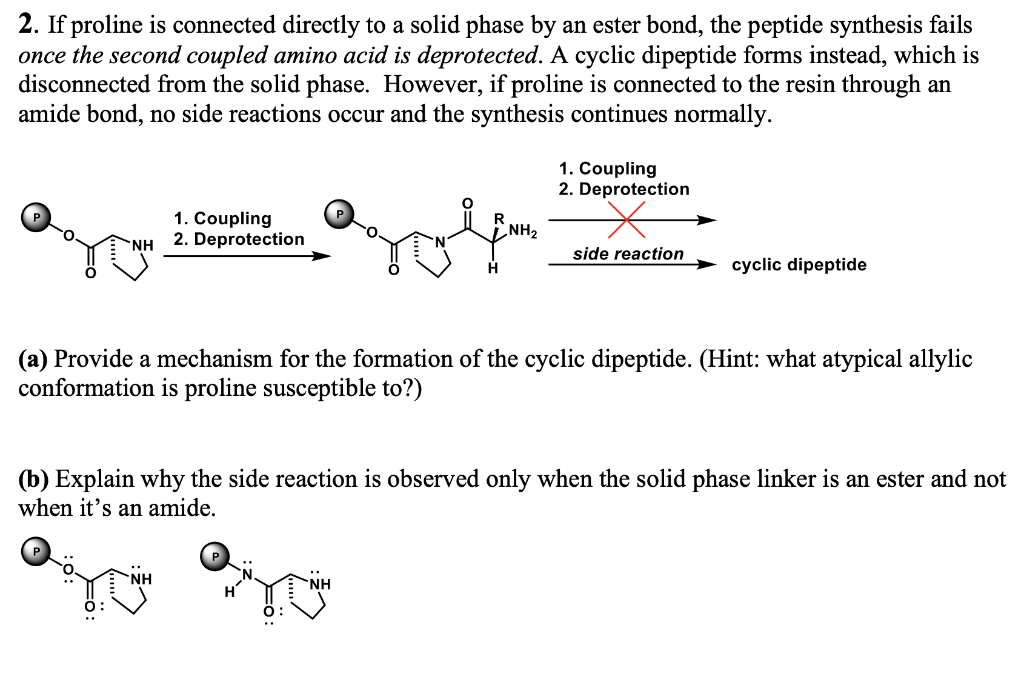
2. If proline is connected directly to a solid phase by an ester bond, the peptide synthesis fails once the second coupled amino acid is deprotected. A cyclic dipeptide forms instead, which is disconnected from the solid phase. However, if proline is connected to the resin through an amide bond, no side reactions occur and the synthesis continues normally. (a) Provide a mechanism for the formation of the cyclic dipeptide. (Hint: what atypical allylic conformation is proline susceptible to?) (b) Explain why the side reaction is observed only when the solid phase linker is an ester and not when it's an amide
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


