Question: Table Q2 (C) below gives some property values for water at 120C and 1.985 bars. Use Table Q2 (C) and with the aid of
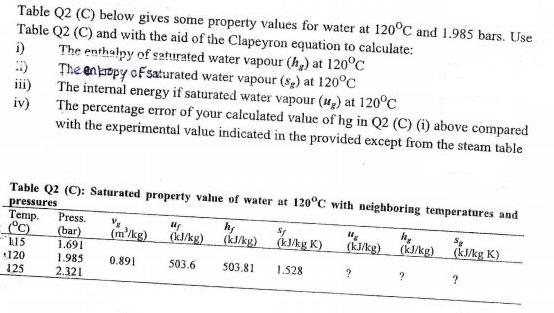
Table Q2 (C) below gives some property values for water at 120C and 1.985 bars. Use Table Q2 (C) and with the aid of the Clapeyron equation to calculate: i) The enthalpy of seturated water vapour (h,) at 120C :) The enkrtpy of saturated water vapour (sp) at 120C iii) The internal energy if saturated water vapour (u,) at 120C iv) The percentage error of your calculated value of hg in Q2 (C) (i) above compared with the experimental value indicated in the provided except from the steam table Table Q2 (C): Saturated property value of water at 120C with neighboring temperatures and pressures Temp. C) Press. (bar) 1.691 (m'/kg) (k/kg) ) (kJkg) (kukg) kJAg K). (k/kg) (kkg K) (kJ/kg K) L15 120 125 1.985 0.891 503.6 503.81 1.528 2.321
Step by Step Solution
3.56 Rating (153 Votes )
There are 3 Steps involved in it
Given Data Temp C Pressure bar vf mkg hf kJkg sf kJkgK uf kJkg 115 1691 5036 50381 120 1985 0891 503... View full answer

Get step-by-step solutions from verified subject matter experts


