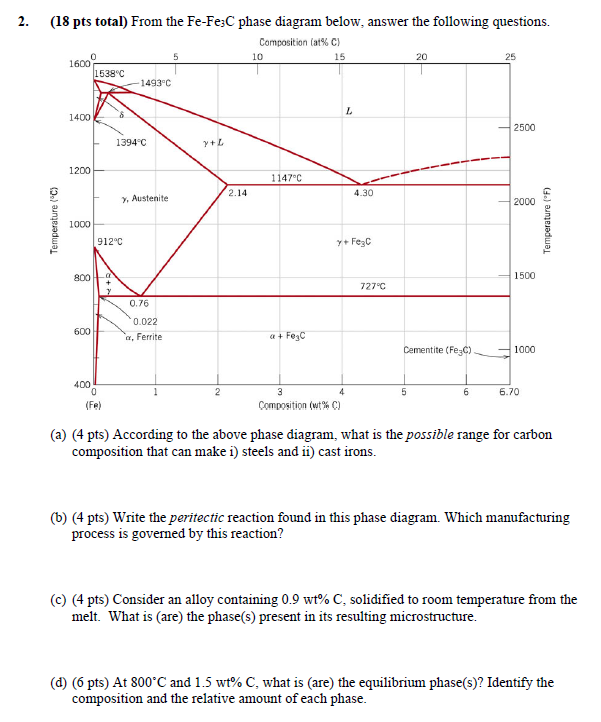
Question: 2 . ( ( mathbf { 1 8 } ) pts total ) From the ( mathrm { Fe }
mathbf pts total From the mathrmFemathrmFemathrmC phase diagram below, answer the following questions.
a pts According to the above phase diagram, what is the possible range for carbon composition that can make i steels and ii cast irons.
b pts Write the peritectic reaction found in this phase diagram. Which manufacturing process is governed by this reaction? please provide a detailed explanation for each step
c pts Consider an alloy containing mathrmwtmathrmC solidified to room temperature from the melt. What is are the phases present in its resulting microstructure.
d pts At circmathrmC and mathrmwtmathrmC what is are the equilibrium phases Identify the composition and the relative amount of each phase.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


