Question: 2. Mean Salt Method Determine the equilibrium constant (solubility constant) when a total of 0.025 m of M2S04 was dissolved when the slightly soluble solid
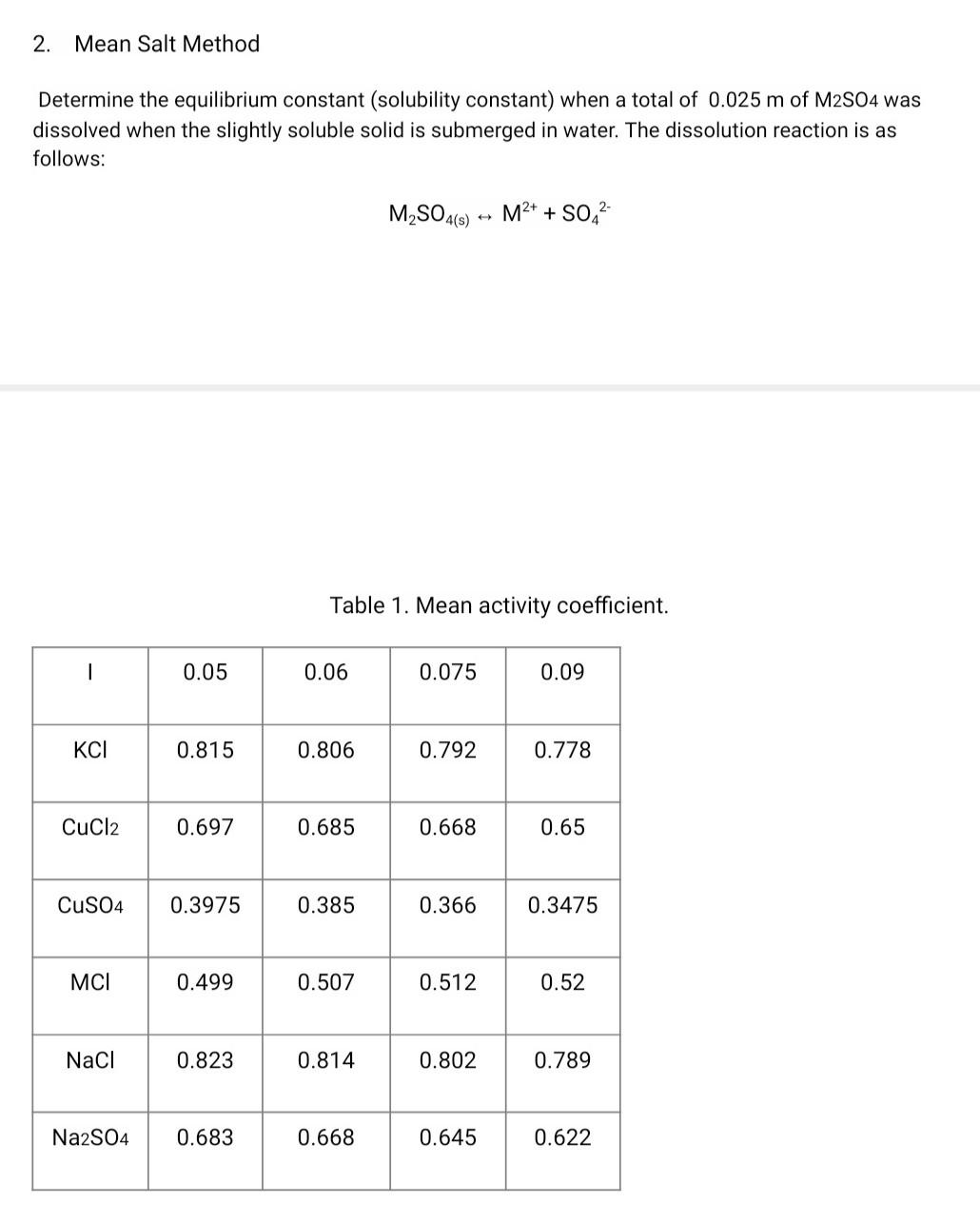
2. Mean Salt Method Determine the equilibrium constant (solubility constant) when a total of 0.025 m of M2S04 was dissolved when the slightly soluble solid is submerged in water. The dissolution reaction is as follows: M2SO4(5) -- M2+ + SO42- Table 1. Mean activity coefficient. 0.05 0.06 0.075 0.09 KCI 0.815 0.806 0.792 0.778 CuCl2 0.697 0.685 0.668 0.65 CuSO4 0.3975 0.385 0.366 0.3475 MCI 0.499 0.507 0.512 0.52 NaCl 0.823 0.814 0.802 0.789 Na2SO4 0.683 0.668 0.645 0.622
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


