Question: 2: need help with all. Just doing one and explaining is fine. 3: I don't know how to use VSEPR theory. 4: last two boxes
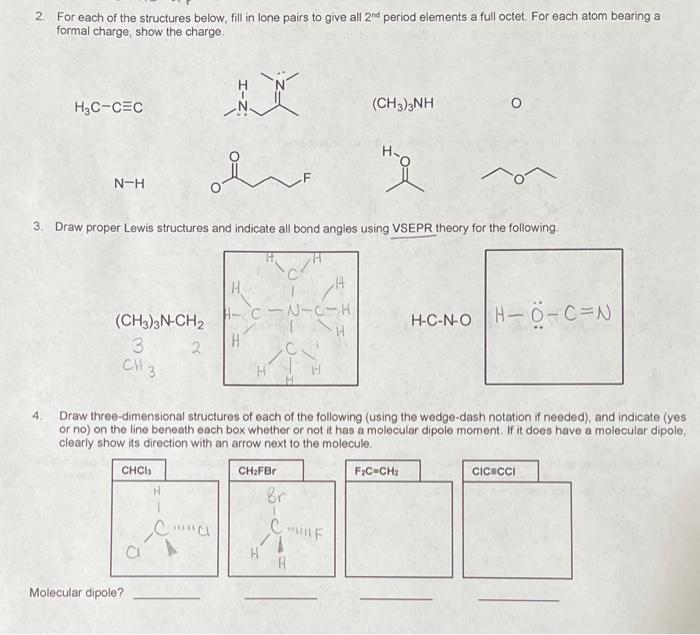
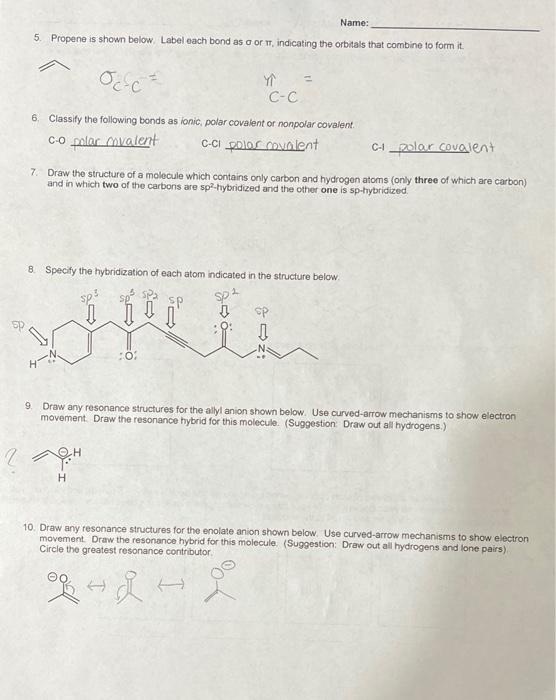
2. For each of the structures below. Fill in lone pairs to give all 2nd period elements a full octet. For each atom bearing a formal charge, show the charge H3C-CEC N. (CH3)2NH N-H 3. Draw proper Lewis structures and indicate all bond angles using VSEPR theory for the following . H A-CN-C-H H (CH3)2N-CH2 3 2 CH3 H-C-N- OH-O-C=N H 4 Draw three-dimensional structures of each of the following using the wedge-dash notation if needed), and indicate (yes or no) on the line beneath each box whether or not it has a molecular dipolo moment. If it does have a molecular dipole, clearly show its direction with an arrow next to the molecule. CHCI FC-CH CICUCCI H Br Ca CHFBr CUF B Molecular dipole? Name: 5. Propene is shown below Label each bond as oor IT, indicating the orbitals that combine to form it. C-c 6. Classify the following bonds as ionic, polar covalent or nonpolar covalent. C-O polac mvalent C-CI polos covalent C-1_zolar covalent 7 Draw the structure of a molecule which contains only carbon and hydrogen atoms (only three of which are carbon) and in which two of the carbons are spa-hybridized and the other one is sp-hybridized 8 Specify the hybridization of each atom indicated in the structure below sp sp SP :01 9 Draw any resonance structures for the allyl anion shown below. Use curved-arrow mechanisms to show electron movement Draw the resonance hybrid for this molecule. (Suggestion Draw out all hydrogens.) 2 H 2 H 10. Draw any resonance structures for the enolate anion shown below. Use curved-arrow mechanisms to show electron movement Draw the resonance hybrid for this moleculo. (Suggestion: Draw out all hydrogens and lone pairs) Circle the greatest resonance contributor og ht
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


