Question: (2 points) Nitrogen fixation is the biological process by which the NN bonds in molecules of atmospheric N2 are broken and nitrogen compounds such an
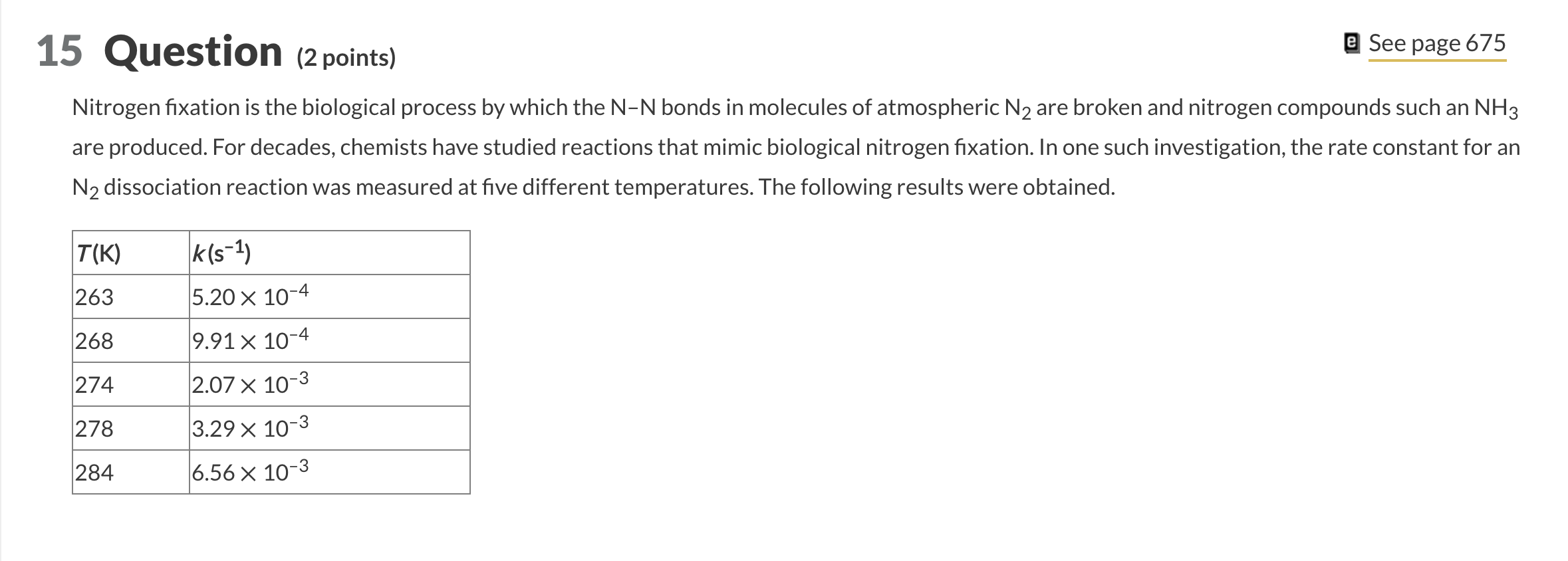
(2 points) Nitrogen fixation is the biological process by which the NN bonds in molecules of atmospheric N2 are broken and nitrogen compounds such an NH3 are produced. For decades, chemists have studied reactions that mimic biological nitrogen fixation. In one such investigation, the rate constant for an N2 dissociation reaction was measured at five different temperatures. The following results were obtained. You will need to plot the data and obtain a trendline to determine the numerical value for this question. What is the activation energy for the reaction? kJ/mol Part 2 What is the value of the rate constant for this reaction at 298K ? s1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


