Question: (2 points) Use the information below to determine whether or not a reaction mixture in which the partial pressures of PCl3,Cl2, and PCl5 are 0.21
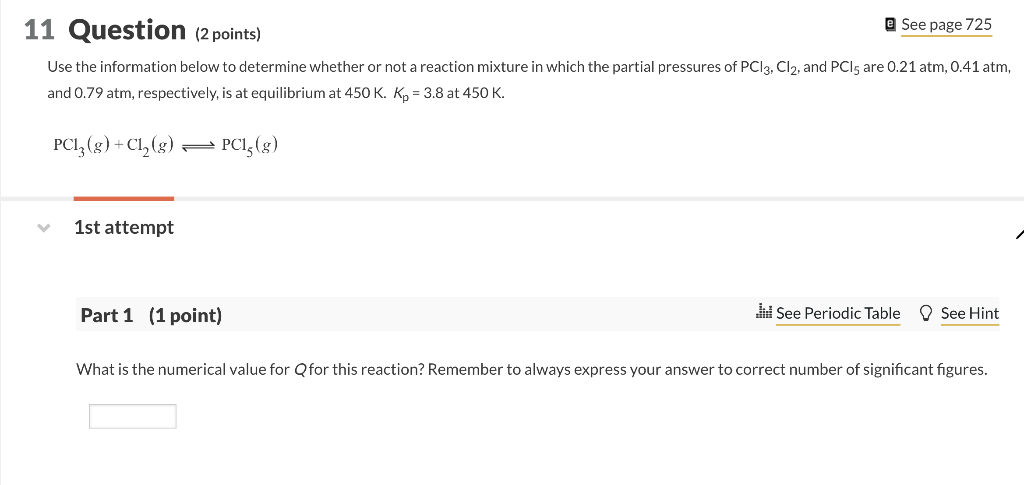

(2 points) Use the information below to determine whether or not a reaction mixture in which the partial pressures of PCl3,Cl2, and PCl5 are 0.21 atm, 0.41 atm, and 0.79atm, respectively, is at equilibrium at 450K.Kp=3.8 at 450K. PCl3(g)+Cl2(g)PCl5(g) 1st attempt Part 1 (1 point) See Periodic Table See Hint What is the numerical value for Q for this reaction? Remember to always express your answer to correct number of significant figures. Formic acid, HCOOH, ionizes in water according to the following equation. The equilibrium constant is K=1.8104. HCOOH(aq)+H2O(l)HCOO(aq)+H3O+(aq) Calculate the equilibrium concentration of H3O+in a 0.985M solution. M
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


