Question: 2. Pure nickel exists in two solid forms Ni(a) (fcc) and Ni(B) (bcc). The enthalpy of transformation from a to B at atmospheric pressure is
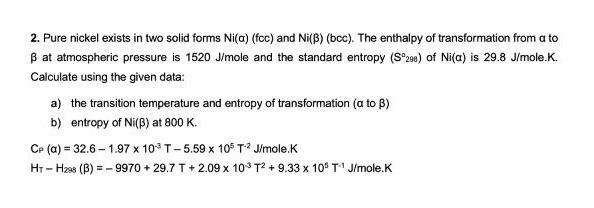
2. Pure nickel exists in two solid forms Ni(a) (fcc) and Ni(B) (bcc). The enthalpy of transformation from a to B at atmospheric pressure is 1520 J/mole and the standard entropy (S29e) of Ni(a) is 29.8 J/mole.K. Calculate using the given data: a) the transition temperature and entropy of transformation (a to B) b) entropy of Ni(f) at 800 K. CP (a) = 32.6 - 1.97 10%T-5.59 x 105 T2J/mole.K H1-H244 (B) = -9970 + 29.7 T + 2.09 x 10$ T2+ 9.33 x 10% T'J/mole.K
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


