Question: 2. Sometimes, E2 can yield 100% Hofmann product, even with a small base. Which of the molecules below will undergo reaction with ethoxide in ethanol
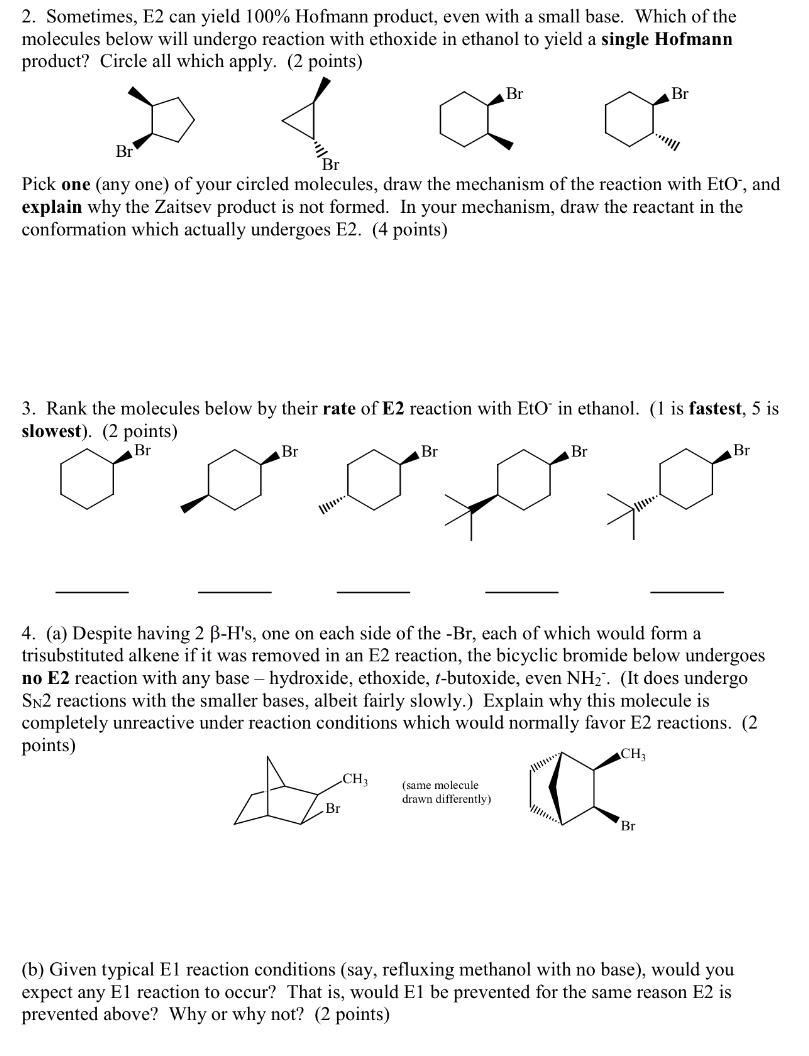
2. Sometimes, E2 can yield 100% Hofmann product, even with a small base. Which of the molecules below will undergo reaction with ethoxide in ethanol to yield a single Hofmann product? Circle all which apply. (2 points) Br Br Br Pick one (any one) of your circled molecules, draw the mechanism of the reaction with Eto', and explain why the Zaitsev product is not formed. In your mechanism, draw the reactant in the conformation which actually undergoes E2. (4 points) 3. Rank the molecules below by their rate of E2 reaction with Eto in ethanol. (1 is fastest, 5 is slowest). (2 points) Br Br Br Br Br 4. (a) Despite having 2 B-H's, one on each side of the -Br, each of which would form a trisubstituted alkene if it was removed in an E2 reaction, the bicyclic bromide below undergoes no E2 reaction with any base - hydroxide, ethoxide, 1-butoxide, even NH". (It does undergo SN2 reactions with the smaller bases, albeit fairly slowly.) Explain why this molecule is completely unreactive under reaction conditions which would normally favor E2 reactions. (2 points) CH CH (same molecule drawn differently Br Br (b) Given typical El reaction conditions (say, refluxing methanol with no base), would you expect any El reaction to occur? That is, would El be prevented for the same reason E2 is prevented above? Why or why not? (2 points)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


