Question: 2. The diagram represents a reversible Carnot cycle for a perfect gas: (a) What is the thermodynamic efficiency of the engine? (b) How much heat
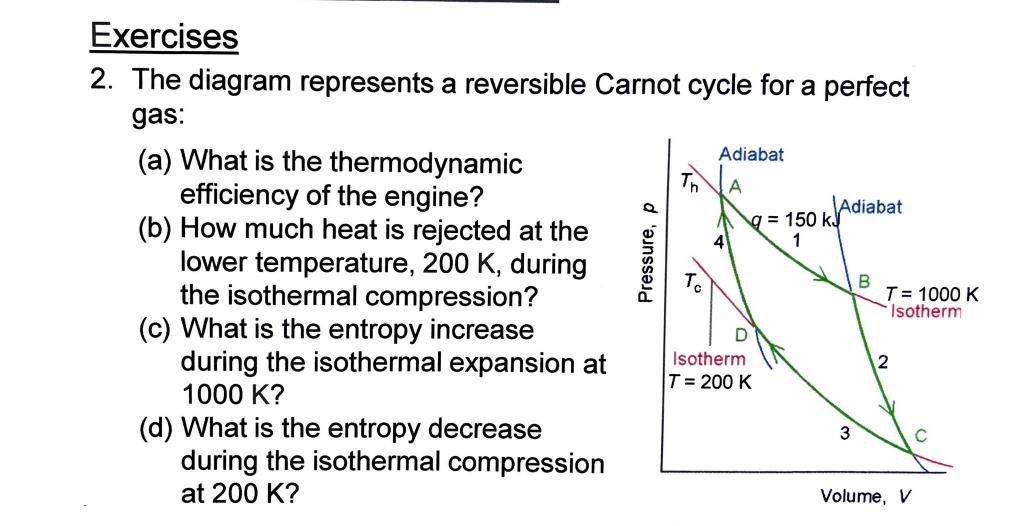
2. The diagram represents a reversible Carnot cycle for a perfect gas: (a) What is the thermodynamic efficiency of the engine? (b) How much heat is rejected at the lower temperature, 200K, during the isothermal compression? (c) What is the entropy increase during the isothermal expansion at 1000K ? (d) What is the entropy decrease during the isothermal compression at 200K
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


