Question: 2. The following information will be derived when we do statistical mechanics. For now, simply accept it as true. The probability a molecule is in
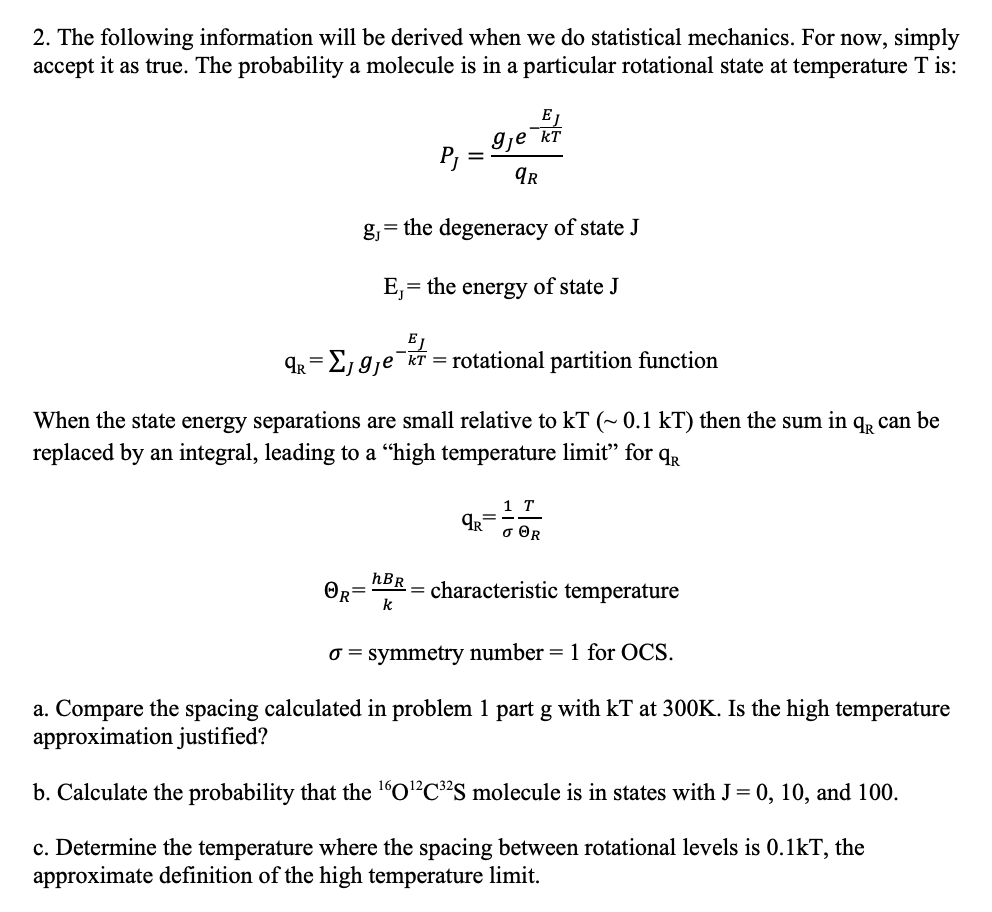
2. The following information will be derived when we do statistical mechanics. For now, simply accept it as true. The probability a molecule is in a particular rotational state at temperature T is: EJ gje KT P = IR = gj= the degeneracy of state J E, = the energy of state J EJ qr = E, gje k = rotational partition function When the state energy separations are small relative to KT (~ 0.1 kT) then the sum in qr can be replaced by an integral, leading to a high temperature limit for qr AR 1 T R HBR OR= -= characteristic temperature k o= symmetry number = 1 for OCS. a. Compare the spacing calculated in problem 1 part g with kt at 300K. Is the high temperature approximation justified? b. Calculate the probability that the 10?C32S molecule is in states with J= 0, 10, and 100. c. Determine the temperature where the spacing between rotational levels is 0.1kT, the approximate definition of the high temperature limit
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


