Question: 2. The ore with the composition given below, is roasted and sulfur (S) amount is reduced to 6.8%.5% of the Cu becomes CuO, and the
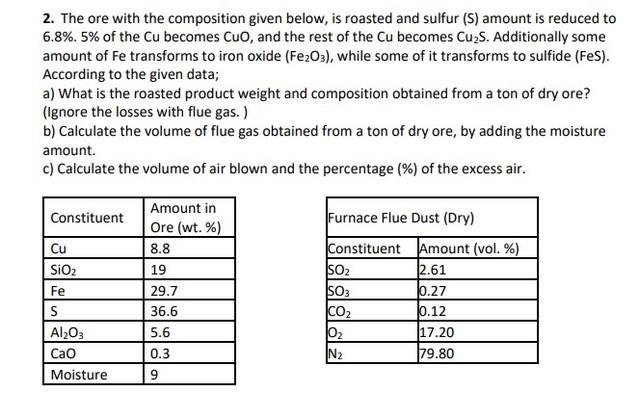
2. The ore with the composition given below, is roasted and sulfur (S) amount is reduced to 6.8%.5% of the Cu becomes CuO, and the rest of the Cu becomes Cu2S. Additionally some amount of Fe transforms to iron oxide ( Fe2O3 ), while some of it transforms to sulfide (FeS). According to the given data; a) What is the roasted product weight and composition obtained from a ton of dry ore? (Ignore the losses with flue gas.) b) Calculate the volume of flue gas obtained from a ton of dry ore, by adding the moisture amount. c) Calculate the volume of air blown and the percentage (\%) of the excess air
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


