Question: 2. There is concern about copper leaching from copper piping into beverages consumed by the population. You want to see if this is indeed something
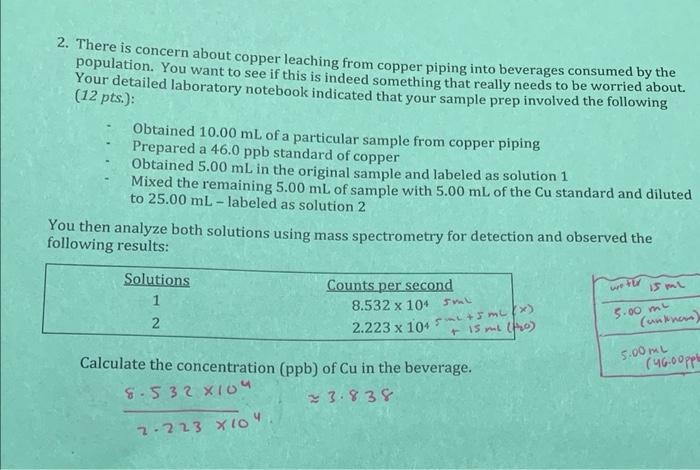
2. There is concern about copper leaching from copper piping into beverages consumed by the population. You want to see if this is indeed something that really needs to be worried about. Your detailed laboratory notebook indicated that your sample prep involved the following (12 pts.): Obtained 10.00 mL of a particular sample from copper piping Prepared a 46.0 ppb standard of copper Obtained 5.00 mL in the original sample and labeled as solution 1 Mixed the remaining 5.00 mL of sample with 5.00 mL of the Cu standard and diluted to 25.00 mL-labeled as solution 2 You then analyze both solutions using mass spectrometry for detection and observed the following results: wetu Solutions 1 2 15 ml 5.00 my (unknom) 2.223 x 104 m) Counts per second 8.532 x 104 cm + 15 m (pro) Calculate the concentration (ppb) of Cu in the beverage. 8.532 X104 23.838 5.00my (46.00pple 2.2.23 X 104
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


