Question: 2. Titanium dioxide is a wide-bandgap semiconductor that is promising as an insulating dielectric in VLSI capacitors and for use in solar cells. Thin films
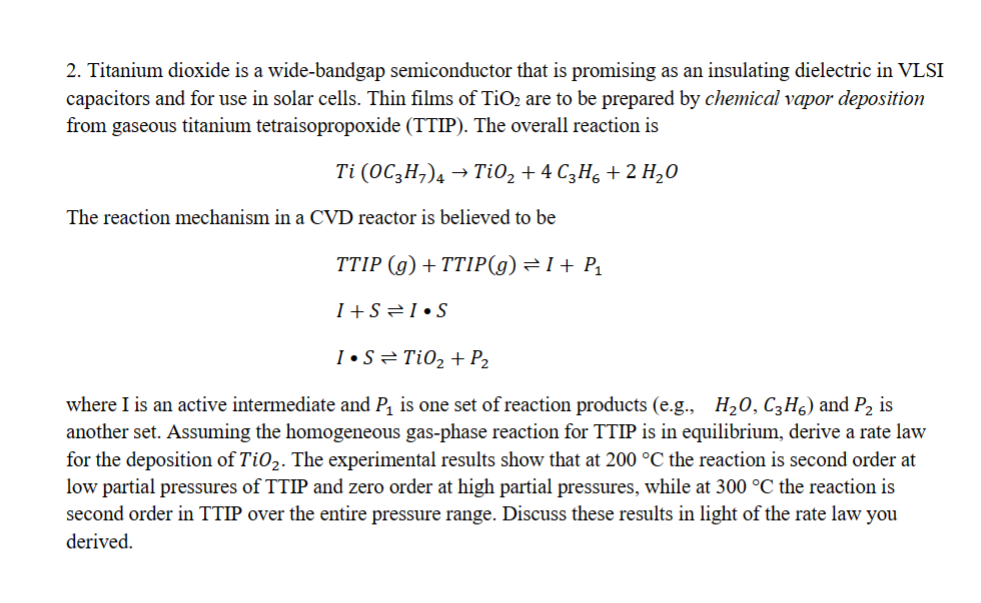
2. Titanium dioxide is a wide-bandgap semiconductor that is promising as an insulating dielectric in VLSI capacitors and for use in solar cells. Thin films of TiO2 are to be prepared by chemical vapor deposition from gaseous titanium tetraisopropoxide (TTIP). The overall reaction is Ti(OC3H7)4TiO2+4C3H6+2H2O The reaction mechanism in a CVD reactor is believed to be TTIP(g)+TTIP(g)I+P1I+SISISTiO2+P2 where I is an active intermediate and P1 is one set of reaction products (e.g., H2O,C3H6 ) and P2 is another set. Assuming the homogeneous gas-phase reaction for TTIP is in equilibrium, derive a rate law for the deposition of TiO2. The experimental results show that at 200C the reaction is second order at low partial pressures of TTIP and zero order at high partial pressures, while at 300C the reaction is second order in TTIP over the entire pressure range. Discuss these results in light of the rate law you derived
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


