Question: 2) Tp Fe is a low-spin Fell) complex with Fe-N bonds of 1.97 . Tp Ph Fe is a similar complex that is high-spin Fe(ll)
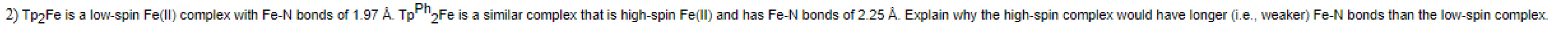
2) Tp Fe is a low-spin Fell) complex with Fe-N bonds of 1.97 . Tp Ph Fe is a similar complex that is high-spin Fe(ll) and has Fe-N bonds of 2.25 A. Explain why the high-spin complex would have longer (i.e., weaker) Fe-N bonds than the low-spin complex
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


