Question: 2. Two reactants, benzyl alcohol and tosyl chloride, react in the presence of an auxiliary, triethylamine, and the solvent toluene to produce the product sulfonate
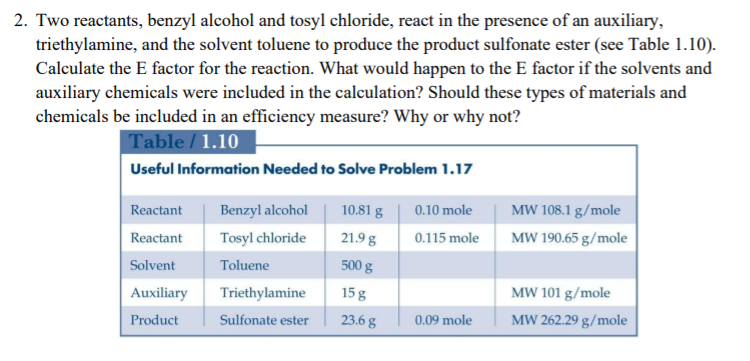
2. Two reactants, benzyl alcohol and tosyl chloride, react in the presence of an auxiliary, triethylamine, and the solvent toluene to produce the product sulfonate ester (see Table 1.10). Calculate the E factor for the reaction. What would happen to the E factor if the solvents and auxiliary chemicals were included in the calculation? Should these types of materials and chemicals be included in an efficiency measure? Why or why not? Table / 1.10 Useful Information Needed to Solve Problem 1.17 0.10 mole MW 108.1 g/mole MW 190.65 g/mole 0.115 mole Reactant Reactant Solvent Auxiliary Product Benzyl alcohol Tosyl chloride Toluene Triethylamine Sulfonate ester 10.81 g 21.98 500 g 15 g 23.6 g MW 101 g/mole MW 262.29 g/mole 0.09 mole
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


