Question: 2. Using the template below, sketch a graph of a perfect separation by distillation of a 40.0 mL solution containing a 50:50 mixture of water
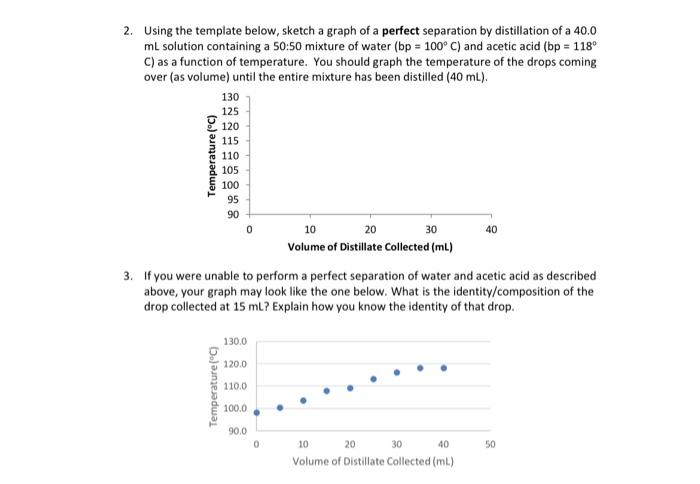
2. Using the template below, sketch a graph of a perfect separation by distillation of a 40.0 mL solution containing a 50:50 mixture of water (bp=100C) and acetic acid (bp=118 C) as a function of temperature. You should graph the temperature of the drops coming over (as volume) until the entire mixture has been distilled ( 40mL). 3. If you were unable to perform a perfect separation of water and acetic acid as described above, your graph may look like the one below. What is the identity/composition of the drop collected at 15mL ? Explain how you know the identity of that drop
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


