Question: 2. Van't Hoff Analysis. In Problem 1 above, you obtained K s at a specific temperature for BDa. In a similar manner K can be
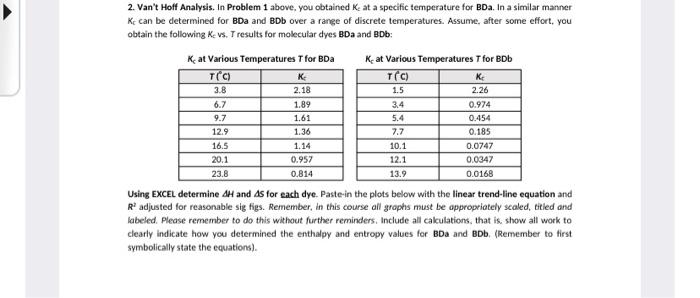

2. Van't Hoff Analysis. In Problem 1 above, you obtained K s at a specific temperature for BDa. In a similar manner K can be determined for BDa and BDb over a range of discrete temperatures. Assume, after some effort, you obtain the following Kvs. T results for molecular dyes BDa and BDb : Kc at Various Temperatures T for BDa K at Various Temperatures T for BDb Using EXCEL determine H and AS for each dye. Paste-in the plots below with the linear trend-line equation and R2 adjusted for reasonable sig figs. Remember, in this course all grophs must be approptiately scaled, titled and labeled. Please remember to do this without further reminders, Include all cakulations, that is. show all work to clearly indicate how you determined the enthalpy and entropy values for BDa and BDb. (Remember to first symbolically state the equations). A1=a1bc1=0.221a1=3.11105cm1m1b=1cmc1=?c1=ba1A1forBDa2(Blue-colour)A2=a2bc2=0.890a2=9.31104cm1m1b=1cmc2=ba2A2substutingKc=BDa1BDa2=A1ba1A2ba2=0.22119.3110410.89013.11105=13.4677
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


