Question: = 2) We know that for an ideal gas Cp,m = Cv,m + R. The general relationship for any gas can be written, for 1
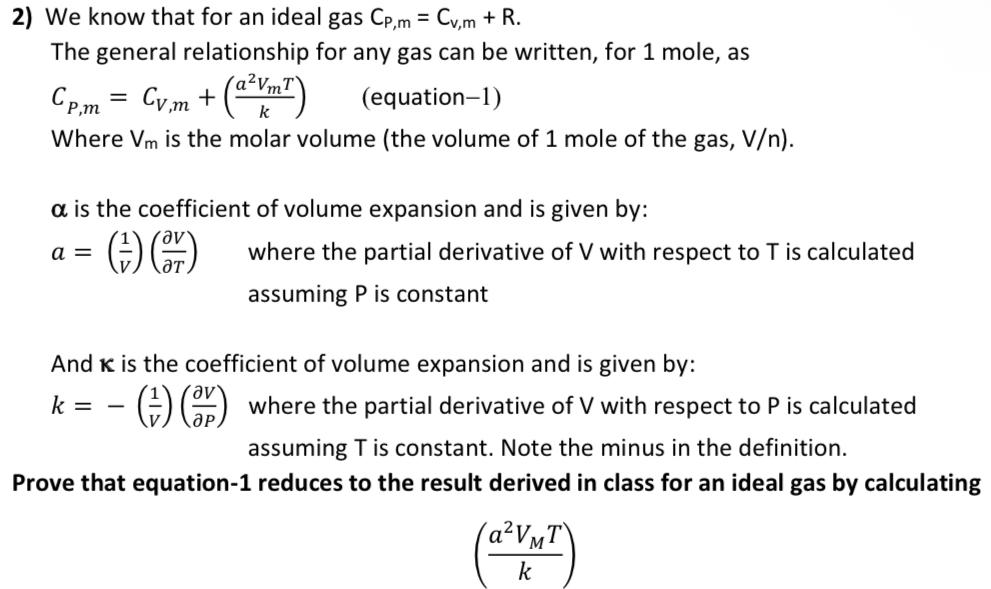
= 2) We know that for an ideal gas Cp,m = Cv,m + R. The general relationship for any gas can be written, for 1 mole, as a (equation-1) Where Vm is the molar volume (the volume of 1 mole of the gas, V). Cp.m = Cv.m + (a?Vm1) k a is the coefficient of volume expansion and is given by: where the partial derivative of V with respect to T is calculated assuming P is constant a = (4) C) ap And K is the coefficient of volume expansion and is given by: k= - (4) ) where the partial derivative of V with respect to P is calculated assuming T is constant. Note the minus in the definition. Prove that equation-1 reduces to the result derived in class for an ideal gas by calculating (aVMT k
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


