Question: (2) Write cell notations for the following cell. (4 Marks) +0.342 V H Solution prepared from 500 ml of 0.010 0 M HOCH 40,0 mL
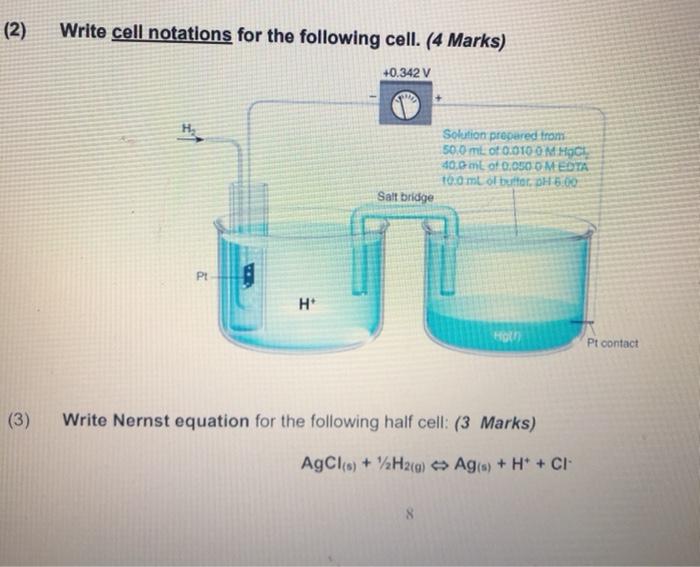
(2) Write cell notations for the following cell. (4 Marks) +0.342 V H Solution prepared from 500 ml of 0.010 0 M HOCH 40,0 mL of 0.050 O MEDIA tomb of buttorH5.00 Salt bridge P! H Pt contact (3) Write Nernst equation for the following half cell: (3 Marks) AgCl(s) + H2(g) Agis) + H+ + CI- 8
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


