Question: $2023 - Chapter 6 - Homework Problem 6.85 - Enhanced - with Feedback begin{tabular}{|l|c|} hline multicolumn{1}{|c|}{ Condition } & Energy(kJ/mol) hlineEca for H(g) &
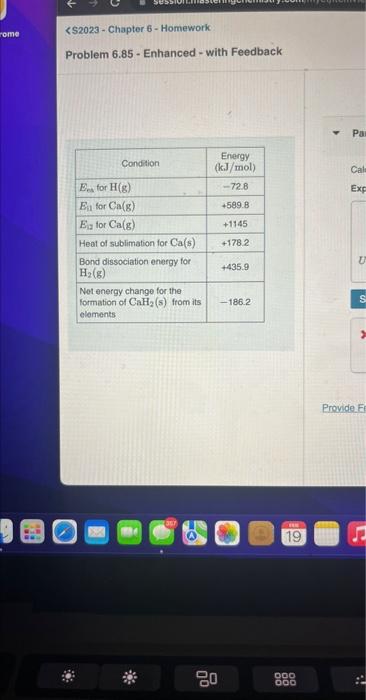
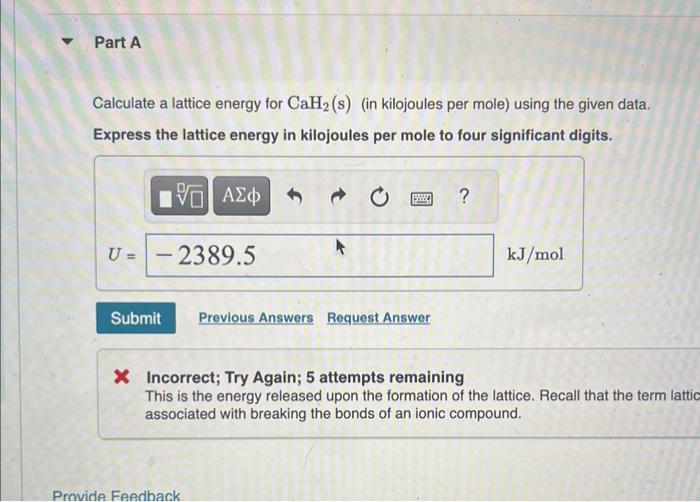
\$2023 - Chapter 6 - Homework Problem 6.85 - Enhanced - with Feedback \begin{tabular}{|l|c|} \hline \multicolumn{1}{|c|}{ Condition } & Energy(kJ/mol) \\ \hlineEca for H(g) & 72.8 \\ \hlineEitforCa(g) & +589.8 \\ \hlineEigforCa(g) & +1145 \\ \hline Heat of sublimation for Ca(s) & +178.2 \\ \hline BonddissociationenergyforH2(g) & +435.9 \\ \hline NetenergychangefortheformationofCaH2(s)fromitselements & 186.2 \\ \hline \end{tabular} Provide. Ff 19 Calculate a lattice energy for CaH2(s) (in kilojoules per mole) using the given data. Express the lattice energy in kilojoules per mole to four significant digits. * Incorrect; Try Again; 5 attempts remaining This is the energy released upon the formation of the lattice. Recall that the term lattic associated with breaking the bonds of an ionic compound
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


