Question: 2-1. Continuous stirred-tank reactors (CSTR) have widespread application in industry and embody many features of other types of reactors. CSTR models tend to be simpler
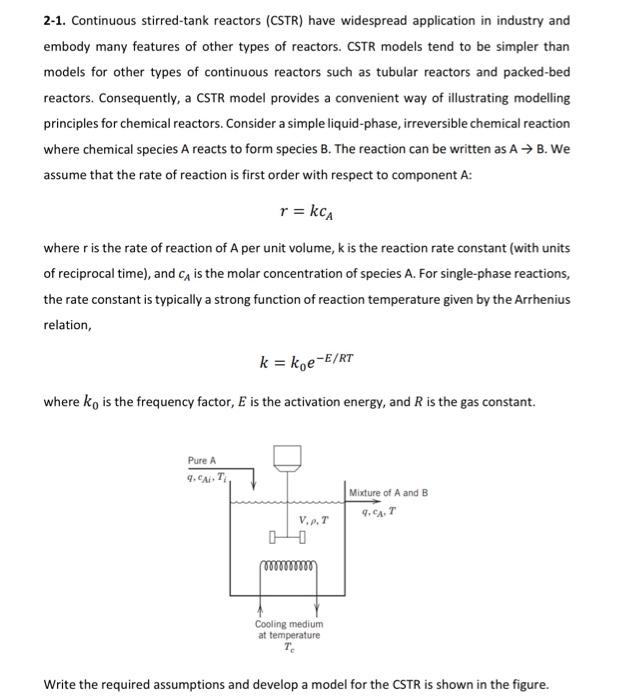
2-1. Continuous stirred-tank reactors (CSTR) have widespread application in industry and embody many features of other types of reactors. CSTR models tend to be simpler than models for other types of continuous reactors such as tubular reactors and packed-bed reactors. Consequently, a CSTR model provides a convenient way of illustrating modelling principles for chemical reactors. Consider a simple liquid-phase, irreversible chemical reaction where chemical species A reacts to form species B. The reaction can be written as A+ B. We assume that the rate of reaction is first order with respect to component A: r = kc where r is the rate of reaction of A per unit volume, k is the reaction rate constant (with units of reciprocal time), and Ca is the molar concentration of species A. For single-phase reactions, the rate constant is typically a strong function of reaction temperature given by the Arrhenius relation, k = koe-E/RT where ko is the frequency factor, E is the activation energy, and R is the gas constant. Pure A 4.CAT Mixture of A and B 4,647 VAT Cooling medium at temperature Write the required assumptions and develop a model for the CSTR is shown in the figure
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


