Question: 2.12 Determine the temperature above or below which the reduction of Mgo by Al becomes thermodynamically feasible at 1 atm pressure (101,325 N/m). Given: 2(A1)
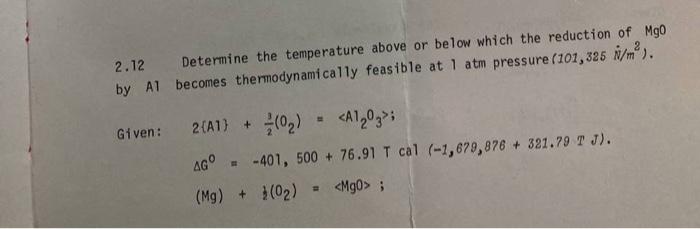
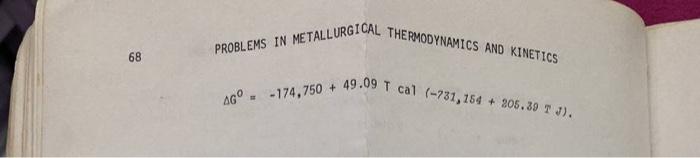
2.12 Determine the temperature above or below which the reduction of Mgo by Al becomes thermodynamically feasible at 1 atm pressure (101,325 N/m). Given: 2(A1) + (0) ; AG THERMODYNAMICS AND KINETICS PROBLEMS IN METALLURGICAL 68 AG -174,750 + 49.09 T cal (-731,154 + 305.39 T J)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


