Question: 2.2e Metal Identification by density Limitations and Applicability in part I of the experiment, an unknown metal will be identified through a determination of its
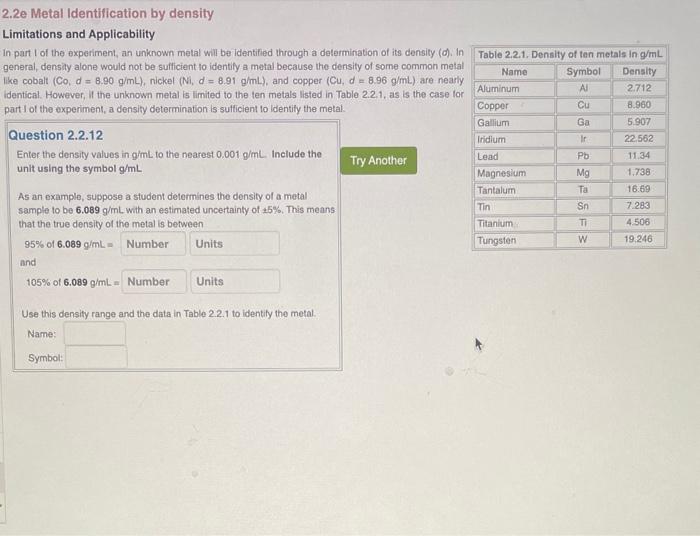
2.2e Metal Identification by density Limitations and Applicability in part I of the experiment, an unknown metal will be identified through a determination of its density (o). In general, density alone would not be sufficient to identify a metal because the density of some common metal like cobalt (Co,d=8.90g/mL), nickel (Ni,d=8.91g/mL), and copper (Cu,d=8.96g/mL) are nearly identical. However, II the unknown metal is limited to the ten metals listed in Table 2.2.1, as is the case tor part I of the experiment, a density determination is sulficient to identify the metal. Question 2.2.12 Enter the density values in gmL to the nearest 0.001g/mL. Include the unit using the symbol g/mL As an example, suppose a student determines the density of a metal sample to be 6.089g/mL with an estimated uncertainty of 5%. This means that the true density of the metal is between 95%of6.089gimL= and 105%of6.089g/mL= Use this density range and the data in Table 2.2.1 to identify the metal. Name: Symbol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


