Question: 23 3 A 2.000-g sample of a solid mixture containing only PbCl2 (FM 278.1), Cuci2 (FM134.45), and KCI (FM 74.55) was dissolved in water to
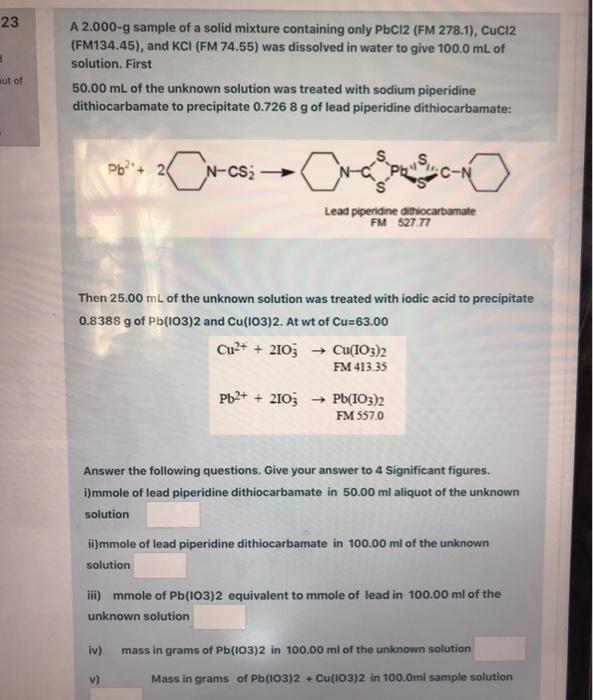

23 3 A 2.000-g sample of a solid mixture containing only PbCl2 (FM 278.1), Cuci2 (FM134.45), and KCI (FM 74.55) was dissolved in water to give 100.0 mL of solution. First 50.00 mL of the unknown solution was treated with sodium piperidine dithiocarbamate to precipitate 0.7268 g of lead piperidine dithiocarbamate: out of Pb2+ 24 C-N Lead piperidine athiocarbamate FM 527.77 Then 25.00 mL of the unknown solution was treated with iodic acid to precipitate 0.8388 g of Pb(103)2 and Cu(103)2. At wt of Cu=63.00 Cu2+ + 2103 Cu(IO3)2 FM 413.35 Pb2+ + 2103 Pb(IO3)2 FM 557.0 Answer the following questions. Give your answer to 4 Significant figures. i)mmole of lead piperidine dithiocarbamate in 50.00 ml aliquot of the unknown solution ii)mmole of lead piperidine dithiocarbamate in 100.00 ml of the unknown solution iii) mmole of Pb(103)2 equivalent to mmole of lead in 100.00 ml of the unknown solution iv) mass in grams of Pb(103)2 in 100.00 ml of the unknown solution Mass in grams of Pb(103)2 + Cu(103)2 in 100.0ml sample solution iii) mmole of Pb(103)2 equivalent to mmole of lead in 100.00 ml of unknown solution iv) mass in grams of Pb(103)2 in 100.00 ml of the unknown solution v) Mass in grams of Pb(103)2 + Cu(103)2 in 100.0ml sample solution vi) Mass in grams of Cu(103)2 in 100.Oml sample solution vii) mmole of Cu(103)2 in 100.0ml sample solution viii) % of Copper in the sample
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


