Question: 23) For the equation: 2 NOBr (g) 2 NO (g) + Br2 (g), you start with 0.75 M of the NOBr. At equilibrium, the
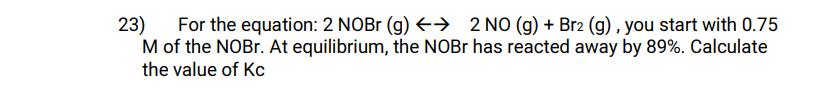
23) For the equation: 2 NOBr (g) 2 NO (g) + Br2 (g), you start with 0.75 M of the NOBr. At equilibrium, the NOBr has reacted away by 89%. Calculate the value of Kc
Step by Step Solution
★★★★★
3.40 Rating (159 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


