Question: 23 What is the pH of a buffer solution composed of 0.125MKF and 0.246MHF ? The Ka of HF is 7.20104 at 25C a. 11.151
23 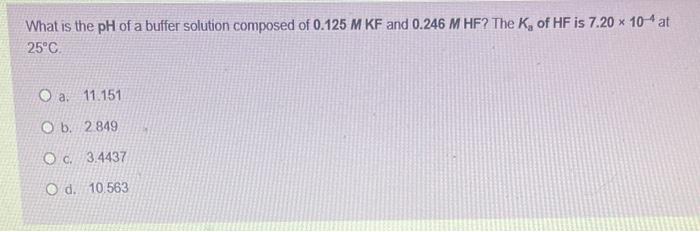

What is the pH of a buffer solution composed of 0.125MKF and 0.246MHF ? The Ka of HF is 7.20104 at 25C a. 11.151 b. 2849 c. 3.4437 d. 10.563
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


