Question: 2.30 L container is filled with methane gas (CH4) at a pressure 3000 mmHg and a temperature of 25 C. How many grams of
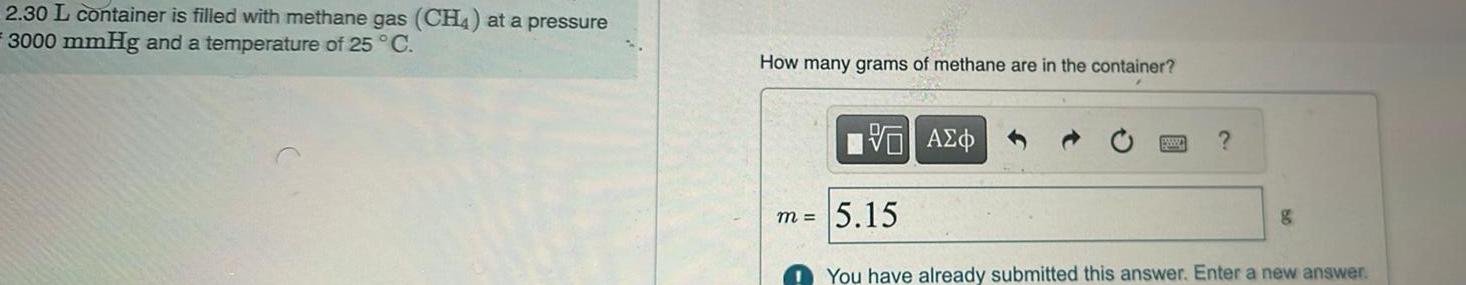
2.30 L container is filled with methane gas (CH4) at a pressure 3000 mmHg and a temperature of 25 C. How many grams of methane are in the container? m = VO ? 5.15 go You have already submitted this answer. Enter a new answer.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


