Question: ? Data A. Precipitation of Ba3(PO4)2 from salt mixture B. Determination of Limiting Reactant Limiting reactant in a salt mixture Na 3 PO4.12H20 Mm =
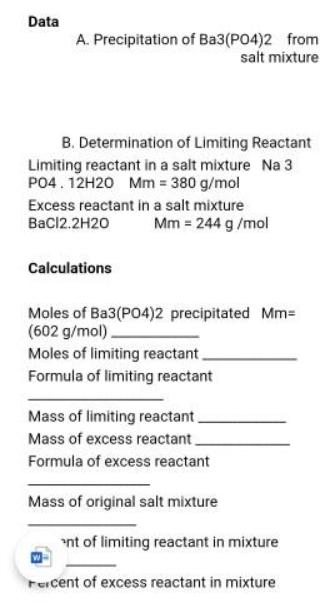 ?
?
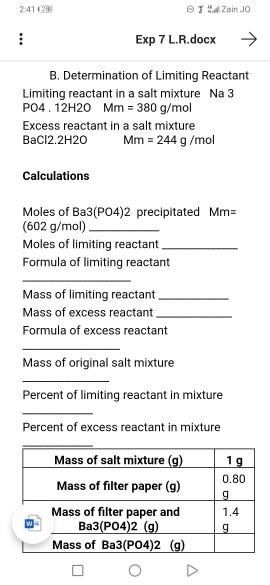
Data A. Precipitation of Ba3(PO4)2 from salt mixture B. Determination of Limiting Reactant Limiting reactant in a salt mixture Na 3 PO4.12H20 Mm = 380 g/mol Excess reactant in a salt mixture BaCl2.2H2O Calculations Mm = 244 g/mol Moles of Ba3(PO4)2 precipitated Mm= (602 g/mol). Moles of limiting reactant. Formula of limiting reactant Mass of limiting reactant. Mass of excess reactant. Formula of excess reactant Mass of original salt mixture nt of limiting reactant in mixture Percent of excess reactant in mixture 2:41 12 Exp 7 L.R.docx B. Determination of Limiting Reactant Limiting reactant in a salt mixture Na 3 PO4. 12H20 Mm = 380 g/mol Excess reactant in a salt mixture BaCl2.2H2O Calculations JZain JO Mm = 244 g/mol Moles of Ba3(PO4)2 precipitated Mm= (602 g/mol). Moles of limiting reactant Formula of limiting reactant Mass of limiting reactant Mass of excess reactant. Formula of excess reactant Mass of original salt mixture Percent of limiting reactant in mixture Percent of excess reactant in mixture Mass of salt mixture (g) Mass of filter paper (g) Mass of filter paper and Ba3(PO4)2 (g) Mass of Ba3(PO4)2 (g) 1g 0.80 g 1.4 g
Step by Step Solution
3.46 Rating (159 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


