Question: 26 Consider the acid-base reaction below: __Fe(OH)2 + __ H3PO4 ----> - Compound A+ - H2O (OR __ F e left parenthesis OH right parenthesis

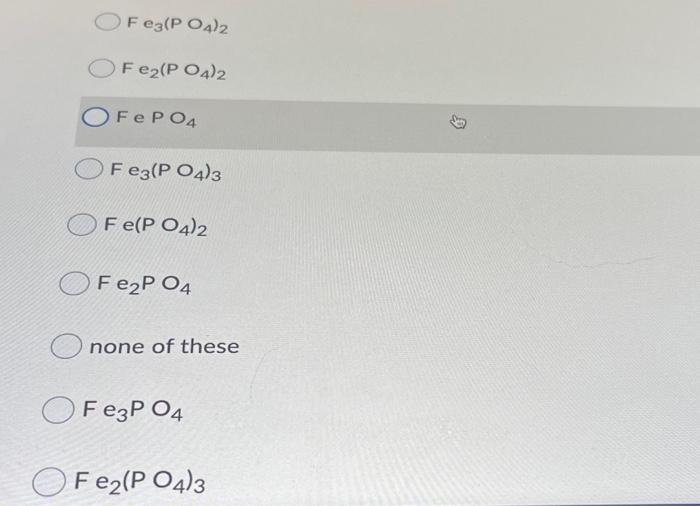
Consider the acid-base reaction below: __Fe(OH)2 + __ H3PO4 ----> - Compound A+ - H2O (OR __ F e left parenthesis OH right parenthesis 2 + _H3PO4 right arrow - Compound A+ _H20) The formula for compound A is OF Fe(PO4)3 Fe3(PO4)2 Fe2(PO4)2 OFePO4 O Fe3(PO4)3 Fe3(PO4)2 Fe2(PO4)2 OFePO4 Fe3(PO4)3 Fe(PO4)2 Fe2P04 none of these FeP 04 O Fe2(PO4)3
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


