Question: 27 Given the following half-reduction reactions: What is Ecell for the following galvanic cell reaction? 3Ag+(s)+Al(s)3Ag(s)+Al3+(2q) a. 0.06V b. +3.02V c. 1.42V d. +1.54V=
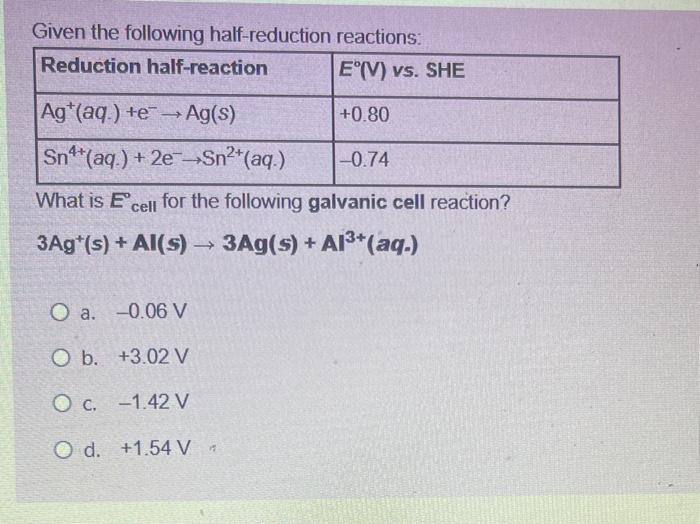
Given the following half-reduction reactions: What is Ecell for the following galvanic cell reaction? 3Ag+(s)+Al(s)3Ag(s)+Al3+(2q) a. 0.06V b. +3.02V c. 1.42V d. +1.54V=
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


