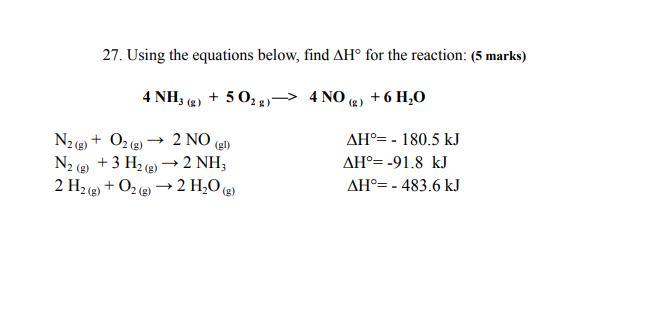
Question: 27. Using the equations below, find AH for the reaction: (5 marks) 4 NH, (2) + 5012)-> 4 NO (2 ) + 6 H,O N2()
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


