Question: 2a. 2b. 2c. 2d. 2e. Which molecule is a secondary alcohol? a. 1-propanol b. 2-methyl-1-hexanol c. 2-methyl-2-hexanol d. 3-methyl-2-hexanol e. more than one correct response
2a.

2b.

2c.
2d.

2e.

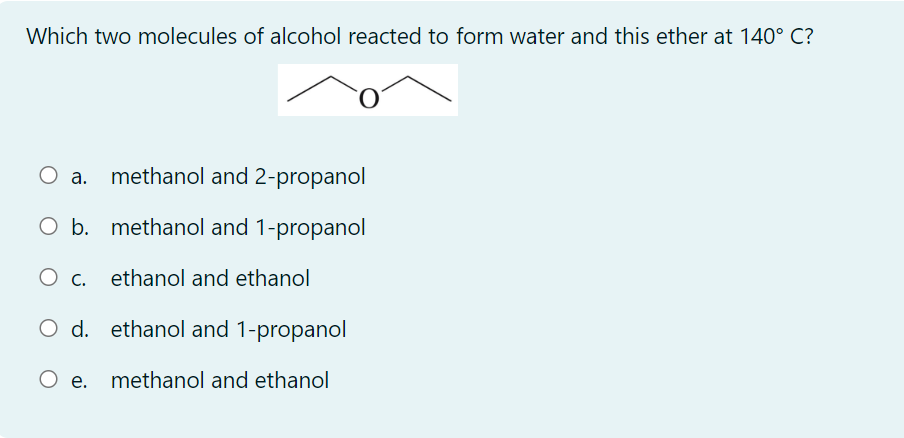
Which molecule is a secondary alcohol? a. 1-propanol b. 2-methyl-1-hexanol c. 2-methyl-2-hexanol d. 3-methyl-2-hexanol e. more than one correct response In which of the following pairs of names do both names represent the same ether? a. diethyl ether and methoxyethane b. methyl isopropyl ether and 1-methoxypropane c. ethyl isopropyl ether and 2-ethoxypropane d. more than one correct response e. no correct response Which two molecules of alcohol reacted to form water and this ether at 140C ? a. methanol and 2-propanol b. methanol and 1-propanol c. ethanol and ethanol d. ethanol and 1-propanol e. methanol and ethanol Which molecule has the highest boiling point? a. CH3CH3 b. CH3OCH3 c. CH3CH2OH d. CH3CH2CH2OCH2CH2CH3 e. CH3CH2CH2CH2CH2OH Which of the following compounds is not an isomer of 2-pentanol? a. 2-methyl-2-butanol b. 1-propoxypropane c. ethyl isopropyl ether d. 2-ethoxypropane e. more than one correct response
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


